- Title
-
Hedgehog signaling is required for differentiation of endocardial progenitors in zebrafish
- Authors
- Wong, K.S., Rehn, K., Palencia-Desai, S., Kohli, V., Hunter, W., Uhl, J.D., Rost, M.S., and Sumanas, S.
- Source
- Full text @ Dev. Biol.
|
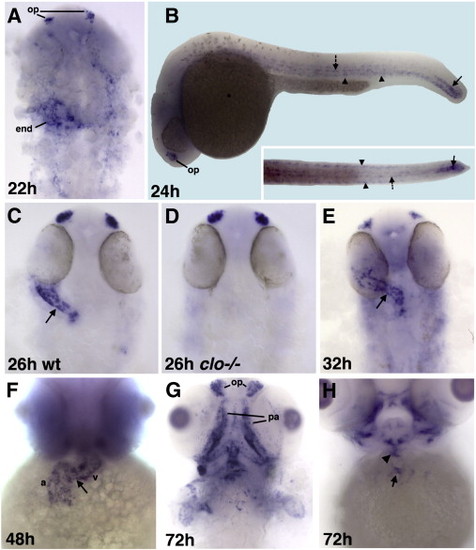
NFATc1 is expressed in the endocardial cells as analyzed by whole-mount in situ hybridization at 22–72 hpf. Dorsal view of flat-mounted embryos, anterior is to the top except as noted. (A) NFATc1 is expressed in the endocardial progenitors (end) and the olfactory placodes (op) at 22 hpf. Much fainter expression is also apparent in the cranial endothelial cells. (B) NFATc1 expression at 24 hpf includes olfactory placodes (op), bilateral subepidermal cells along the trunk (arrowheads), adaxial cells along the midline (dotted arrow), the most caudal part of the notochord and the tailbud (arrow). Lateral (the main panel) and dorsal views of the trunk and tail regions (inset). Endocardial expression is not apparent in these views. (C,D) Endocardial NFATc1 expression (arrow, C) is absent in clo-/- mutants (D) compared with their siblings in (C) as analyzed at 26 hpf. Approximately µ (16 out of 63) embryos obtained from heterozygous clo+/- carriers showed this phenotype. (E,F) NFATc1 expression throughout the endocardium at 32 hpf (E) and 48 hpf (F) (a, atrium; v, ventricle; arrow points to the atrioventricular boundary). (G,H) NFATc1 expression at 72 hpf includes the olfactory placodes (op) and pharyngeal arch skeleton (pa). NFATc1 is no longer expressed throughout the endocardium but is localized to the valve-forming regions in the atrioventricular canal (arrow, H) and the outflow tract (arrowhead, H). EXPRESSION / LABELING:
PHENOTYPE:
|
|
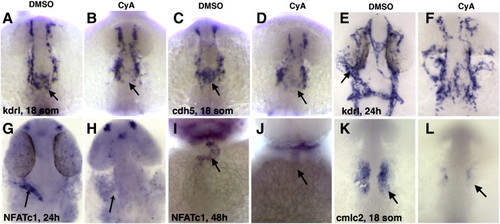
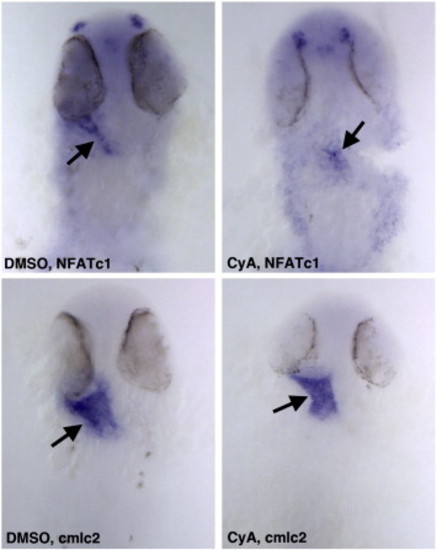
Endocardial and myocardial markers are reduced or absent in cyclopamine (CyA) treated embryos compared to dimethylsulfoxide (DMSO)-treated controls. (A–D) Kdrl (A,B) and cdh5 (C,D)-expression within endocardial progenitors that migrate to the midline (arrow) is greatly reduced in CyA-treated embryos (B,D) compared to controls (A,C) at the 18-somite stage. Dorsal view of the anterior region, anterior is up. (E–J) Endocardial kdrl (E,F) and NFATc1 (G-J) expression (arrows) is greatly reduced or absent in CyA-treated embryos (F,H,J) compared to controls (E,G,I) at the 24 h (E–H) and 48 h (I,J) stages. (K,L) Myocardial cmlc2 expression (arrows) is reduced in CyA-treated embryos at the 18-somite stage. (E–L) Ventral view of deyolked flat-mounted embryos, anterior is up. EXPRESSION / LABELING:
|
|
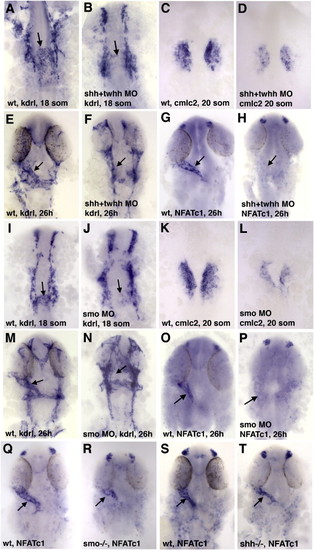
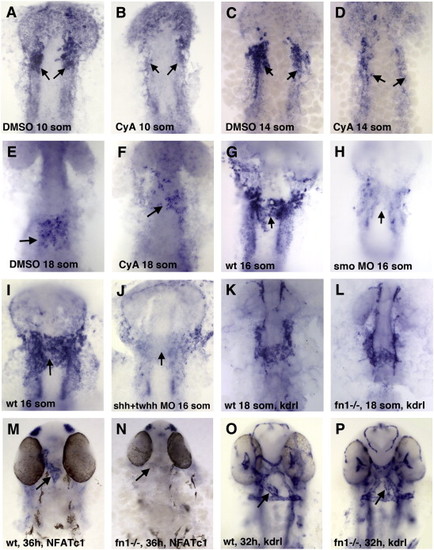
Endocardial and myocardial markers are reduced in shh + twhh and smo MO knockdown and mutant embryos. Ventral view of the anterior region in flat-mounted embryos except as noted; anterior is to the top. (A–H) Injection of a mixture of shha and twhh/shhb MOs resulted in strong downregulation of endocardial kdrl (A,B,E,F), NFATc1 (G,H) (arrows) and myocardial cmlc2 (C,D) expression. (I–P) Smo MO injection results in the reduction or absence of kdrl-expressing endocardial progenitors at the midline at the 18-somite stage (arrows, I,J), the absence of endocardial kdrl (arrows, M,N) and NFATc1 expression at 26 hpf (arrows, O,P) and reduction of myocardial cmlc2 expression at the 20-somite stage (K,L). (Q–T) Smo-/- (R) and shha-/- (T) genetic mutant embryos display reduced endocardial NFATc1 expression (arrows) compared to their siblings (Q,S). EXPRESSION / LABELING:
|
|
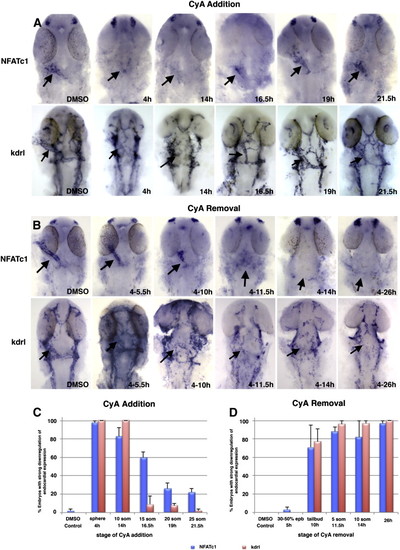
Hedgehog signaling is required during 10–16.5 hpf period for endocardial differentiation based on NFATc1 expression analysis at 26 hpf. Flat-mounted embryos, dorsal view, anterior is to the top. (A) Cyclopamine addition at different stages of development shows that Hh signaling is required at least until approximately 16.5 hpf for endocardial NFATc1 and kdrl expression (arrows) while treatment after 19 hpf has little effect on the endocardium formation. Left panels, control embryos incubated in DMSO. Note the absence of endocardial tube in the embryos treated starting at 4 and 14 hpf. (B) Cyclopamine removal at different stages of development shows that Hh signaling is required from 10 hpf for endocardial NFATc1 and kdrl expression (arrows). Numbers indicate the treatment interval. (C,D) Percentage of embryos that display significant NFATc1 (blue bars) and kdrl (red bars) reduction (more than 50% of expression area is affected) at different stages of cyclopamine addition (C) and removal (D). EXPRESSION / LABELING:
|
|
CyA treatment at the 15-somite stage selectively affects endocardial NFATc1 but not myocardial cmlc2 expression. Embryos were treated in CyA solution starting from the 15-somite stage until 25 hpf and analyzed at 25 hpf stage. Ventral view of flat-mounted embryos, anterior is to the top. EXPRESSION / LABELING:
|
|
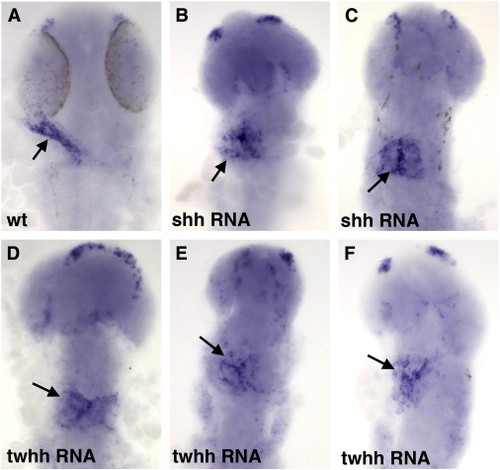
Overexpression of Hh results in a failure of endocardial progenitors to coalesce into the heart tube (arrows) as analyzed by NFATc1 expression at 26 hpf. (A) Control uninjected embryo, (B,C) shh and (D–F) twhh RNA injected embryos. |
|
Hh signaling is required for the initiation of fibronectin (fn1) expression in the ALPM region while fn1 function is required for endocardial differentiation. (A–F) Fn1 expression in the endocardial progenitors (arrows) is reduced in CyA-treated embryos (B,D,F) compared to DMSO-treated controls (A,C,E) at the 10-somite (A,B), 14-somite (C,D) and 18-somite (E,F) stages. (G–J) Fn1 expression is absent from the endocardial progenitors in smo MO (H) and shh + twhh MO mixture (J) -injected embryos as compared with uninjected controls (G,I) at the 16-somite stage. (K–P) Analysis of endocardial markers in nat/fn1-/- mutant embryos. (K,L) Endocardial kdrl expression is present at the midline in nat/fn1 homozygous mutant embryos (L), similar to their wild-type siblings at the 18-somite stage (K). (M,N) Endocardial NFATc1 expression is absent in fn1/nat mutants at 36 hpf. (O,P) Endocardial kdrl expression appears to be mislocalized and reduced in fn1/nat mutants at 32 hpf. Note that while the arrow points to the presumptive endocardial tube in nat mutants (P), it is not possible to reliably distinguish endocardial from vascular endothelial cells based on kdrl expression alone. All panels, ventral view of flat-mounted embryos, anterior is up. Individual nat mutant embryos were genotyped at the 18-somite stage following in situ hybridization as described previously (Garavito-Aguilar et al., 2010), while at older stages they were separated based on their morphological phenotypes. EXPRESSION / LABELING:
PHENOTYPE:
|
|
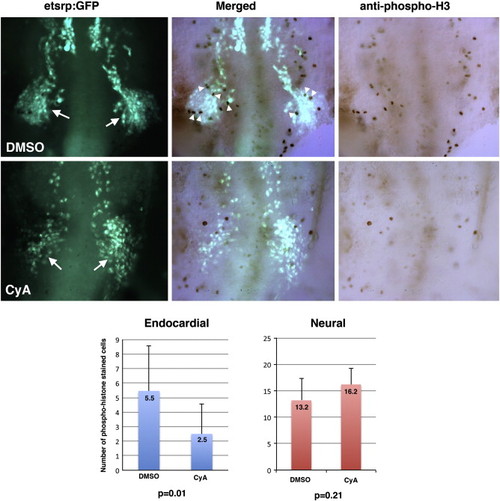
Proliferation of endocardial progenitors is reduced in CyA-treated embryos compared to DMSO-treated controls. Maximum projection images of Tg(etsrp:EGFP) fluorescence (left), phospho-histone staining (right) and overlap of the two channels (middle) are shown. Ventral views of flattened deyolked embryos at the 13-somite stage, anterior is to the top. Formation of the midbrain-organizing center (MOC, arrows) which gives rise to many cranial endothelial, endocardial and myeloid progenitors is not significantly affected in CyA-treated embryos. However, phospho-histone staining within the MOC region (arrowheads) is significantly reduced in CyA-treated embryos. Note that because the presented view is the maximum projection image, staining in some cells may appear to overlap while they are actually present in different focal planes. Left graph shows average cell count per embryo of phospho-histone stained Tg(etsrp:EGFP) cells in the MOC region which is significantly different between DMSO and CyA-treated embryos. As shown in the right graph, the number of neural progenitors scored in a randomly selected area is not significantly different between the DMSO and CyA-treated embryos. |
|
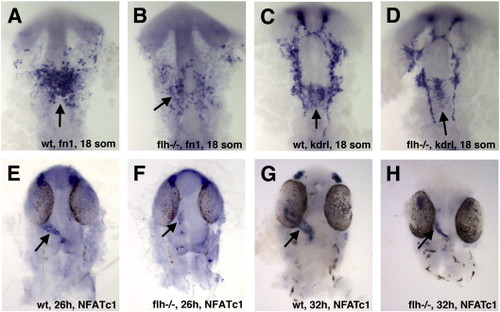
Endocardial morphogenesis is inhibited in floating head (flh) mutants. (A–D) The number of endocardial fn1 (A,B) and kdrl (C,D) -expressing precursors is reduced in the notochord deficient flh-/- mutant embryos as compared to their phenotypically normal siblings at the 18-somite stage. (E–H) NFATc1 expression is reduced in flh mutants at 24hpf (E,F) and 32 hpf (G,H) stages. In all panels, ventral view of flat-mounted embryos, anterior is to the top. EXPRESSION / LABELING:
PHENOTYPE:
|
|
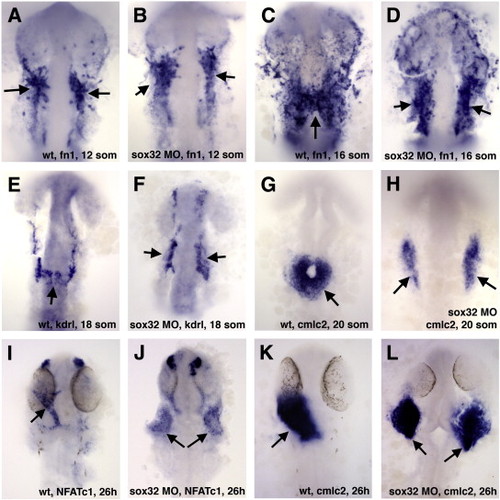
Sox32 function is required for the migration but not differentiation of endocardial precursors. (A–D) Fn1 expression in the endocardial precursors is not affected in the endoderm-deficient sox32 knockdown embryos at the 12-somite stage (A,B) while they fail to migrate towards the midline at the 16-somite stage (C,D). (E,F) Endocardial kdrl expression is absent from the midline in sox32 morphants at the 18-somite stage. (G–L) Myocardial cmlc2 (G,H,K,L) and endocardial NFATc1 expression (I,J) is split bilaterally in sox32 morphants at the 20-somite (G,H) and 26 hpf stages (I-L). Ventral view of flat-mounted embryos, anterior is to the top in all panels. EXPRESSION / LABELING:
PHENOTYPE:
|
|
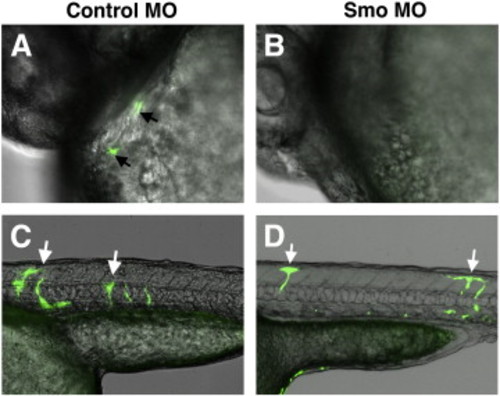
Donor cells from smo MO-injected Tg(fli1a:EGFP) embryos contribute to vascular endothelial but not endocardial lineage. Cells were transplanted from smo MO or control MO-injected Tg(fli1a:EGFP) embryos into wt recipients. Overlap of bright field and green channels, anterior is to the left, embryos are at 32 hpf. (A,B) Endocardial cells from Tg(fli1a:EGFP) control donor embryos (arrows, A) contribute at a greater frequency to the endocardium of wt recipient embryos compared to the cells from smo MO donors (B). Note that while two fluorescent cells are shown in (A), the majority of recipient embryos that showed any contribution of Tg(fli1a:EGFP) cells to the endocardium, displayed only a single GFP-positive cell. (C,D) Transplanted cells from both control and smo morphants contribute to vascular endothelium (arrows). |
|
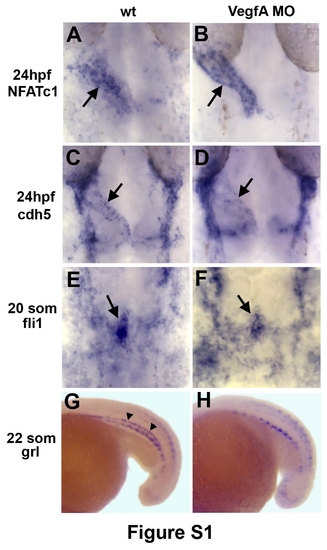
VegfA MO knockdown does not affect endocardial differentiation while arterial differentiation is perturbed. (A-F) Endocardial (arrows) NFATc1 (A,B), cdh5 (C,D) and fli1 expression (E,F) is not significantly affected in VegfA MO injected embryos. Dorsal view of flat-mounted embryos, anterior is to the top. (G,H) Arterial grl expression (arrowheads, G) is absent in Vegf morphants, which confirms the efficacy of Vegf MO. Lateral view of the trunk region, anterior it to the left. The remaining grl expression in Vegf morphants is non-vascular. |
|
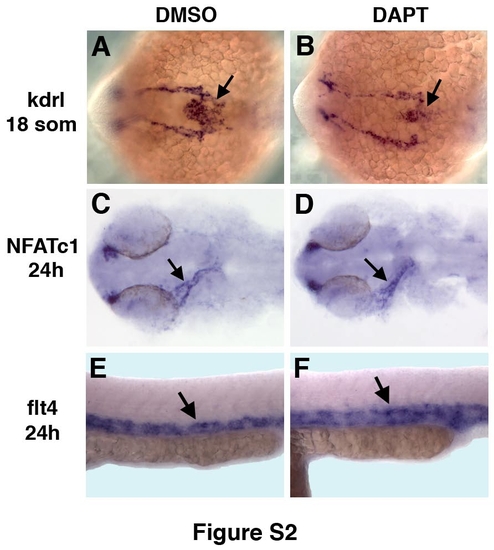
Inhibition of Notch signaling by DAPT treatment does not significantly affect endocardial morphogenesis while arterial-venous differentiation is perturbed. (A-D) Endocardial kdrl (arrows, A,B) and NFATc1 (arrows, C,D) expression at the 18-somite and 24 hpf stages, respectively, is not affected in DAPT-treated embryos (B,D) compared to DMSO-treated controls (A,C). (E,F) Venous flt4 expression at 24 hpf (arrow) is expanded in DAPT treated embryos (F) compared to DMSO-treated controls (E). |
|
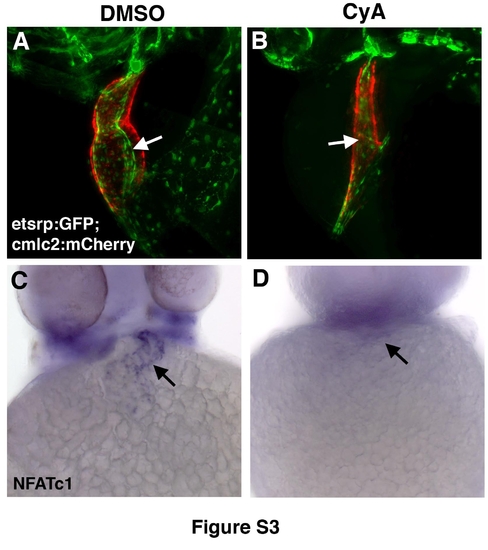
Reduced endocardium is present in CyA-treated embryos at 48hpf while NFATc1 expression is absent. (A,B) Endocardium and myocardium visualized by etsrp:GFP (green) and cmlc2:mCherry (red) expression in live DMSO control (A) and CyA-treated embryos (B). Note the presence of reduced myocardium and endocardium and the absence of heart looping in CyA-treated embryos. (C,D) Endocardial NFATc1 expression is absent in CyAtreated embryos (D) as compared to DMSO-treated controls (C). All panels, ventral view of whole-mounted embryos, anterior is to the top. (A,B) Maximal projection images were obtained using compound fluorescence microscopy (Zeiss, AxioImager) and subjected to 3Ddeconvolution (AutoQuant software package). |
|
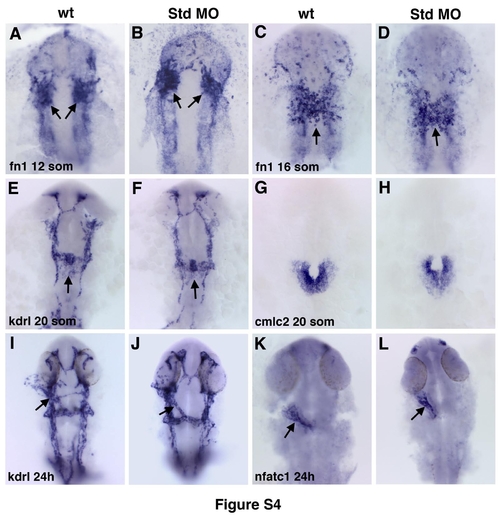
Injection of standard control morpholino does not affect endocardial or myocardial morphogenesis. Endocardial (arrows) fn1 (A-D), kdrl (E,F,I,J), nfatc1 (K,L) and myocardial cmlc2 (G,H) expression is not significantly affected in standard MO-injected embryos compared to uninjected controls. |
|
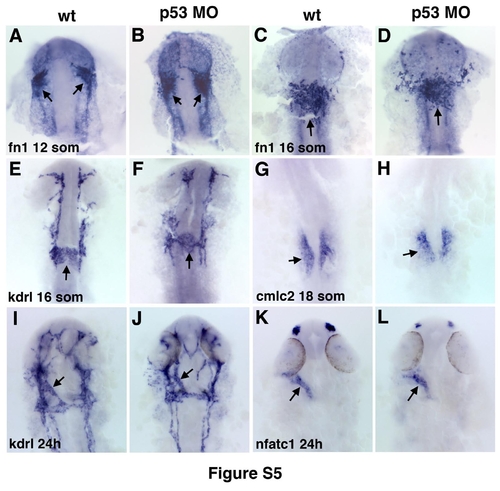
Injection of p53 morpholino does not affect endocardial or myocardial morphogenesis. Endocardial (arrows) fn1 (A-D), kdrl (E,F,I,J), nfatc1 (K,L) and myocardial cmlc2 (G,H) expression is not significantly affected in p53 MO-injected embryos compared to uninjected controls. |
|
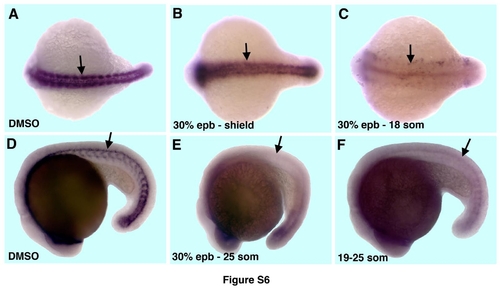
CyA treatment results in strong reduction in ptc1 expression. (A-C). Neural ptc1 expression at the 18-somite stage (arrows) is not affected in the embryos that were CyA-treated from the 30% epiboly to the shield stage (4.5-6hpf, B) but is nearly completely absent in the embryos that were continuously incubated in CyA-solution from the 30% epiboly stage (C) as compared to DMSO-treated controls (A). Dorsal view, anterior is to the left. (D-F) CyA-treatment from the 19-somite stage (F) results in a similar reduction in ptc1 expression (arrows) as the treatment from the 30% epiboly stage (E) as compared to DMSOtreated controls (D). Embryos at the 25-somite stage, lateral view, anterior is to the left. |
|
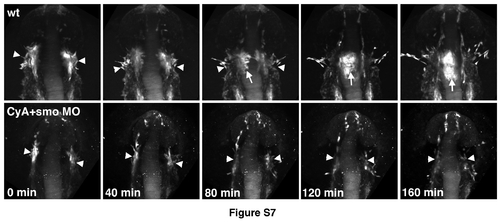
Time-lapse images of endocardial formation in etsrp:GFP control and CyAtreated smo morphant embryos. Embryos were imaged starting from the 11-12-somite stage. Dorsal view of the cranial region, anterior is up. Etsrp:GFP-expression labels vascular endothelial, myeloid and endocardial lineages that originate primarily in the midbrain organizing center (MOC, arrowheads). Note that MOC formation is not significantly affected in CyA+smo MO embryos (0 min). Endocardial progenitors (arrow) migrate to the midline in wt embryos while this migration is largely absent in CyA+smo morphants. |
|
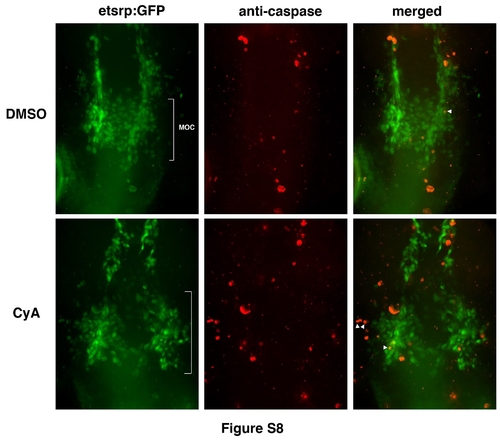
CyA treatment does not result in increased apoptosis among endocardial progenitors. Overlay (right panels) of etsrp:GFP (left) and anti-caspase staining (middle) at the 14-15-somite stages. Maximal projection images are shown. Note that multiple apoptotic and etsrp:GFP cells are in different Z-planes from cells although they may appear to overlap in the projection image. Overlapping staining is shown in white arrowheads. Anti-caspase stained cells were quantified in the MOC region (while brackets). As analyzed by Student’s t-Test, the average numbers and standard deviation of anti-caspase stained etsrp:GFP cells per embryo were 4.1±3.2 (n=11) and 6.0±3.9 (n=8) in DMSO control and CyA-treated samples, respectively, which is not statistically different (p=0.25). |
Reprinted from Developmental Biology, 361(2), Wong, K.S., Rehn, K., Palencia-Desai, S., Kohli, V., Hunter, W., Uhl, J.D., Rost, M.S., and Sumanas, S., Hedgehog signaling is required for differentiation of endocardial progenitors in zebrafish, 377-91, Copyright (2012) with permission from Elsevier. Full text @ Dev. Biol.