- Title
-
Semaphorin7A patterns neural circuitry in the lateral line of the zebrafish
- Authors
- Dasgupta, A., Reagor, C.C., Paik, S.P., Snow, L.M., Jacobo, A., Hudspeth, A.J.
- Source
- Full text @ Elife
|
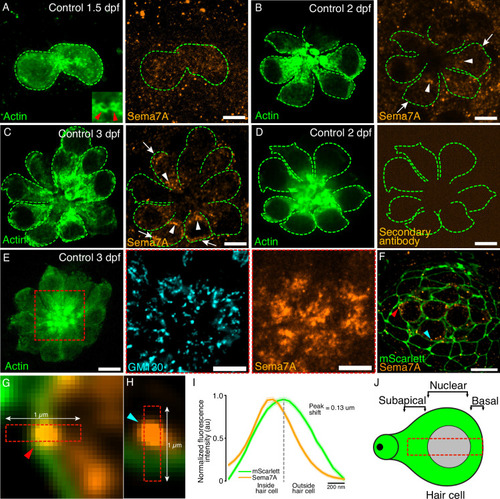
Expression of Semaphorin7A in the zebrafish’s lateral line. (A) A volumetric rendering of a PLL neuromast depicts the sensory axons (magenta) that branch from the lateral line nerve to arborize around the basolateral surface of the hair cell cluster (green). Additional cell types in the neuromast are not labeled. Larval age, 3 dpf. A=anterior, P=posterior, D=dorsal, V=ventral. (B) A schematic drawing of the two variants of the Sema7A protein molecule depicts the full-length GPI-anchored form and the smaller, potentially secreted form. Both the molecules include a signal sequence (green) and a conserved sema domain (orange). (C) Tg(myo6b:actb1-EGFP) larvae at 4 dpf were terminally anesthetized and their heads were removed with fine blades. The tails were then dissociated into the component cells, which were sorted by flow cytometry. Green cells represent GFP+ hair cells. A cDNA library was prepared from the isolated RNA. (D) Gel-based RT-PCR analysis indicates the presence of both the sema7a transcript variants and the expression of hair cell-specific genes, parvalbumin 8 (pvalb8) and S100 calcium binding protein T (s100t). (E) A surface micrograph of a neuromast at 3 dpf depicts two pairs of mature hair cells (green dashed lines) and a pair of immature hair cells (grey dashed lines). Inset: among the three pairs of hair cell apices, the immature pair is indicated by arrowheads. Immunolabeling reveals that the Sema7A protein (orange) occurs consistently at the subapical region (arrowheads) and at the basolateral surface (arrows) of a hair cell. In this and in each of the subsequent neuromast images, anterior is to the left and dorsal to the top. (F) A plot quantifies developmental changes in the average Sema7A intensity in both rostrally and caudally polarized hair cells of neuromasts from 1.5 dpf to 4 dpf. The data stem from 18, 36, 39, and 40 hair cells in neuromasts of respectively 1.5 dpf, 2 dpf, 3 dpf, and 4 dpf larvae. (G) A plot quantifies the distribution of average Sema7A intensity along the hair cell’s apicobasal axis. The results stem from 52, 57, and 54 hair cells of neuromasts from 2 dpf, 3 dpf, and 4 dpf larvae. (H) An immunofluorescence image at the nuclear level of a 4 dpf neuromast shows the contact of the sensory arbors (magenta) with the basolateral surface of the hair cells (green). Immunolabeling for Sema7A (orange) reveals enrichment of the protein at the hair cell bases and sensory-axon interfaces (arrowheads). (I) At the base of the hair cell, association of the td-Tomato+ sensory arbors positively correlated with the Sema7A intensity. The results stemmed from 48 hair cells of 13 neuromasts from 3 dpf larvae. HC, hair cell; scale bars, 5 µm; au, arbitrary unit; means ± SEMs; ρ, Spearman’s correlation coefficient; *** implies p<0.001 and ** implies p<0.01. |
|
Isolation of GFP-expressing hair cells. ( |
|
Expression of Semaphorin7A in the lateral line neuromast. ( |
|
Sema7A progressively enriches at the hair cell base with the sensory axons. (A) A micrograph at a nuclear plane of a 3 dpf neuromast depicts several actin-GFP (green) labeled hair cells. The lateral (white dashed line) and the basal (green dashed line) surfaces of three hair cells are outlined. (B) The td-Tomato-positive sensory arbors contact the basal regions (green dashed line) of three hair cells. (C) The Sema7A is enriched in the basal regions (green dashed line) of the hair cells that are in contact with the sensory arbors. (D, E) At the base of the hair cell, intensities of the td-Tomato-positive sensory arbors positively correlate with the Sema7A intensities. The results stem from 66 hair cells of 15 neuromasts from 2 dpf larvae and 40 hair cells of 11 neuromasts from 4 dpf larvae. Scale bars, 5 µm; au, arbitrary unit; ρ, Spearman’s correlation coefficient. |
|
Alignment of the mouse and the zebrafish Sema7A protein sequences generated by Clustal Omega. ( |
|
sema7a-/- mutants display aberrant sensory axon arborizations. (A–D) In micrographs of 3 dpf control and sema7a-/- neuromasts, Myosin VI (green) marks the hair cells (green dashed lines). The level of Sema7A (orange) is highly reduced in the sema7a-/- mutant compared to the control, with sporadic localization in the subapical region (arrowheads) and none in the basolateral (arrow) region. (E) A plot of normalized Sema7A intensity from 99 control and 100 sema7a-/- hair cells quantitates the effect. (F, G) Surface views of a control and a sema7a-/- neuromast at 4 dpf depict the interaction of the sensory arbors (magenta) with hair cell clusters (green). In the control, the arbors intimately contact the hair cells, with a few exceptions (arrowhead). In the sema7a-/- mutant, the arbors direct many aberrant projections (arrowheads) away from the hair cell cluster. The two immature hair cell pairs in the sema7a-/- neuromast are indicated by arrows. (H,I) Skeletonized networks portray the three-dimensional topology of the sensory arbors from the control and the sema7a-/- neuromasts depicted in panels F and G, respectively. The pseudocolored trajectories depict the increase in arbor contour length from each point of arborization, defined as the point at which the lateral line branch (magenta) contacts hair cell cluster. (J, K) Micrographs depict the skeletonized networks of the combined 4 dpf hair cell clusters, whose centers are located at (0,0). The X- and Y-coordinates represent the anteroposterior (AP) and the dorsoventral (DV) axes of the larva, respectively. Positive values of the X- and Y-coordinates represent the posterior and ventral directions, respectively. Combined skeletonized network traces from 27 control and 27 sema7a-/- mutant neuromasts are represented. (L) The plot denotes the densities of the sensory arbors around the center of the combined hair cell clusters for 35 control (magenta) and 27 sema7a-/- (red) neuromasts at 4 dpf. The shaded area marks the region proximal to the boundary of the combined hair cell cluster. (M) The plot quantifies the degree of contact of the sensory arbors to their hair cell clusters in individual neuromasts from both control (magenta) and sema7a-/- mutants (red), each point represents a single neuromast. Thirty-three, 29, and 35 neuromasts were analyzed from 2 dpf, 3 dpf, and 4 dpf control larvae, respectively. Seventeen, 53, and 27 neuromasts were analyzed from 2 dpf, 3 dpf, and 4 dpf sema7a-/- mutant larvae, respectively. HC, hair cell; Scale bars, 5 µm; au, arbitrary unit; means ± SEMs; *** signifies p<0.001; ** signifies p<0.01. |
|
( |
|
Quantification of sensory arbor distributions and contacts with hair cell clusters. ( |
|
Sema7A patterns the topology of the sensory arbor network. (A) A schematic drawing of the combined hair cell cluster (green) and associated sensory axons (magenta) shows diverse topological features of the arborization network, including nodes, loops, bare terminals, node degree, and bare-terminal curvature. The apex is defined as the center of the hair bundle cluster and the base is denoted by the node from which the arborizations extends. The relative organ height describes the position along the axis formed between the arborization initiation point and the apex of the hair cell cluster. (B) The correlation between the relative number of bare terminals and loops from 35 control neuromasts and 27 sema7a-/- neuromasts at 4 dpf shows a negative correlation in the control but a scattered distribution in the mutants. The same neuromasts were analyzed in the subsequent panels. R2, correlation coefficient. Dashed red line depicts the regression slope. (C) The relationship of contour lengths to bare terminals and loops reveals reduced looping in the mutants. (D) Quantification of the number of nodes per loop demonstrates a decrease in mutants. (E) Mutant larvae manifest a broader distribution of loop lengths compared to controls. (F) A schematic diagram of the extending bare terminals depicts regions of high and low curvature along the length of the arbor. The distributions of bare terminals between low- and high-curvature groups show an enrichment of long, highly curved arbors in the control and short, linear arbors in the mutants. Statistical analyses are displayed in Table 1. |
|
Quantification of topological features across developmental stages and genotypes. ( |
|
Quantification of topological features between control and ( |
|
Ectopic Sema7Asec diffusive cue provides neural guidance in vivo. (A) A diagrammatic overview depicts the generation of a transgenic animal that expresses the Sema7Asec protein ectopically under the control of a thermally inducible promoter. Larvae with ectopic myotomal-integration near the network of sensory arbors (red rectangle) were imaged to analyze arbor morphology. (B) A schematic drawing of a sensory arbor from a heat-shocked larva depicts an extended axonal projection (arrowhead) that reaches toward the myofibers expressing the ectopic Sema7Asec protein. Parameters that quantitate the accuracy of the extended axonal projections toward the ectopic Sema7Asec sources are denoted. (C) In a micrograph of an ectopically expressing Sema7Asec (orange) larva, the sensory arbor (magenta) extends two aberrant axonal projections. One elongates (cyan arrowhead) along the somite boundary to reach and contact an ectopically integrated muscle progenitor cell (white dashed line) and the other (red arrowhead) reenters the posterior lateral-line nerve while following a second ectopic source. The through-focus scans (i–iii) from the epidermis to the dermomyotome and the three-dimensional (3D) surface reconstruction (iv) reveal the intimate contact between the aberrant sensory arbor (arrowheads) and the muscle progenitor cell. (D) In a micrograph of an ectopically expressing Sema7Asec (orange) larva, the sensory arbor (magenta) extends a single aberrant axonal process (cyan arrowhead) to reach ectopically integrated myofibers (white dashed line). The through-focus scans (i–iii) from the epidermis to the dermomyotome and the 3D surface reconstruction (iv) reveal the proximal association of the aberrant sensory arbor (arrowheads) to the myofibers. Melanocytes (yellow arrowhead) along the horizontal myoseptum intermittently block the visibility of the lateral-line nerve. (E) An injected, but not heat-shocked, control larva does not express ectopic Sema7Asec and does not show aberrant projection from the sensory arbor. (F) A plot demonstrates the accuracy of 18 extended axonal arbors in finding ectopic Sema7Asec sources. Each circle represents a single ectopic integration event. The two pairs of numbers represent the minimal and maximal lengths of the projection path (black) and its corresponding source path (green). (G) A plot quantitates the distribution of projection-proximity length from 18 ectopic integration events. (H) A schematic drawing of a section of the lateral-line nerve between two neuromasts from a heat-shocked larva depicts a few extended neurites (magenta) reaching toward the cells expressing the ectopic Sema7Asec protein (orange). The Sema7Asec integration site (blue rectangle) with the sensory axonal projections was imaged for ten hours to visualize axonal dynamics. (I) Micrographs (i-iii) at three distinct times in the time-lapse video microscopy show directed branching (cyan arrowheads) of the sensory neurites from the lateral-line nerve toward the ectopic Sema7Asec sources. Each panel depicts the ectopic integration site and the associated sensory neurites from three different planes, XY, XZ, and YZ. White arrowheads in XZ and YZ planes highlight the close association of the sensory axons with the ectopic Sema7Asec sources. The t=0 hr denotes the onset of imaging, which is two hours post the beginning of heat shock. (J) The micrographs depict in pseudocolored trajectories the dwell times of the sensory neurites (left) at the Sema7Asec sources (right) for ten hours. MPC, muscle progenitor cell; scale bars, 20 µm; means ± SEMs. |
|
Ectopic Sema7Asec diffusive cues induce aberrant sensory neurite extension. ( |
|
Ectopic integration of Sema7Asec distant from the neuromast induces sensory neurite extension. ( |
|
Ectopic Sema7A-GPI fails to guide sensory arbors from a distance. ( |
|
Ectopic Sema7Asec diffusive cue provides neural guidance in vivo. (A) A diagrammatic overview depicts the generation of a transgenic animal that expresses the Sema7Asec protein ectopically under the control of a thermally inducible promoter. Larvae with ectopic myotomal-integration near the network of sensory arbors (red rectangle) were imaged to analyze arbor morphology. (B) A schematic drawing of a sensory arbor from a heat-shocked larva depicts an extended axonal projection (arrowhead) that reaches toward the myofibers expressing the ectopic Sema7Asec protein. Parameters that quantitate the accuracy of the extended axonal projections toward the ectopic Sema7Asec sources are denoted. (C) In a micrograph of an ectopically expressing Sema7Asec (orange) larva, the sensory arbor (magenta) extends two aberrant axonal projections. One elongates (cyan arrowhead) along the somite boundary to reach and contact an ectopically integrated muscle progenitor cell (white dashed line) and the other (red arrowhead) reenters the posterior lateral-line nerve while following a second ectopic source. The through-focus scans (i–iii) from the epidermis to the dermomyotome and the three-dimensional (3D) surface reconstruction (iv) reveal the intimate contact between the aberrant sensory arbor (arrowheads) and the muscle progenitor cell. (D) In a micrograph of an ectopically expressing Sema7Asec (orange) larva, the sensory arbor (magenta) extends a single aberrant axonal process (cyan arrowhead) to reach ectopically integrated myofibers (white dashed line). The through-focus scans (i–iii) from the epidermis to the dermomyotome and the 3D surface reconstruction (iv) reveal the proximal association of the aberrant sensory arbor (arrowheads) to the myofibers. Melanocytes (yellow arrowhead) along the horizontal myoseptum intermittently block the visibility of the lateral-line nerve. (E) An injected, but not heat-shocked, control larva does not express ectopic Sema7Asec and does not show aberrant projection from the sensory arbor. (F) A plot demonstrates the accuracy of 18 extended axonal arbors in finding ectopic Sema7Asec sources. Each circle represents a single ectopic integration event. The two pairs of numbers represent the minimal and maximal lengths of the projection path (black) and its corresponding source path (green). (G) A plot quantitates the distribution of projection-proximity length from 18 ectopic integration events. (H) A schematic drawing of a section of the lateral-line nerve between two neuromasts from a heat-shocked larva depicts a few extended neurites (magenta) reaching toward the cells expressing the ectopic Sema7Asec protein (orange). The Sema7Asec integration site (blue rectangle) with the sensory axonal projections was imaged for ten hours to visualize axonal dynamics. (I) Micrographs (i-iii) at three distinct times in the time-lapse video microscopy show directed branching (cyan arrowheads) of the sensory neurites from the lateral-line nerve toward the ectopic Sema7Asec sources. Each panel depicts the ectopic integration site and the associated sensory neurites from three different planes, XY, XZ, and YZ. White arrowheads in XZ and YZ planes highlight the close association of the sensory axons with the ectopic Sema7Asec sources. The t=0 hr denotes the onset of imaging, which is two hours post the beginning of heat shock. (J) The micrographs depict in pseudocolored trajectories the dwell times of the sensory neurites (left) at the Sema7Asec sources (right) for ten hours. MPC, muscle progenitor cell; scale bars, 20 µm; means ± SEMs. |
|
( |
|
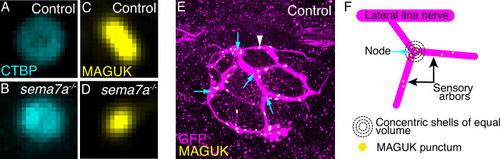
Morphologies of presynaptic and postsynaptic assemblies and the distribution of postsynaptic aggregates across the sensory arbor network. ( |
|
Expression of diverse neural guidance cues in developing neuromasts. Analysis of single cell RNA-sequencing data ( |