- Title
-
Positive and negative regulation of Gli activity by Kif7 in the zebrafish embryo
- Authors
- Maurya, A.K., Ben, J., Zhao, Z., Lee, R.T., Niah, W., Ng, A.S., Iyu, A., Yu, W., Elworthy, S., van Eeden, F.J., and Ingham, P.W.
- Source
- Full text @ PLoS Genet.
|
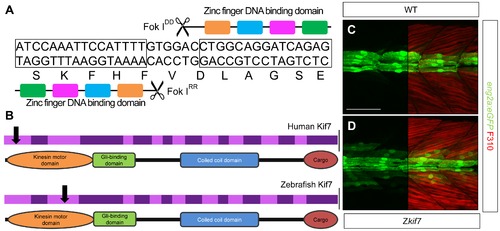
Targeted mutation of the zebrafish kif7 gene. (A) Schematic representation of the nucleotide sequence in exon 3 of the zebrafish kif7 gene targeted by the Zinc finger nucleases. (B) Schematic representation of the human and zebrafish Kif7 coding sequences showing conserved exonic structure (dark shading) corresponding to different protein domains (drawn to scale). The black arrows indicate the approximate position of homozygous viable mutations found in some human patients and of the induced lesions in the zebrafish gene. (C,D) Expression of eng2a:gfp reporter gene in muscle fibers in the tail somites of wild-type (C) and kif7 homozygous (D) embryos at 2.5 dpf. Low level ectopic expression of the reporter is detected in fibers surrounding the muscle pioneers in the kif7 mutants. Merged images showing fast twitch muscle fibers stained with mAb F310 (red) are shown in the right-hand panels. Scale bar: 50 μm. |
|
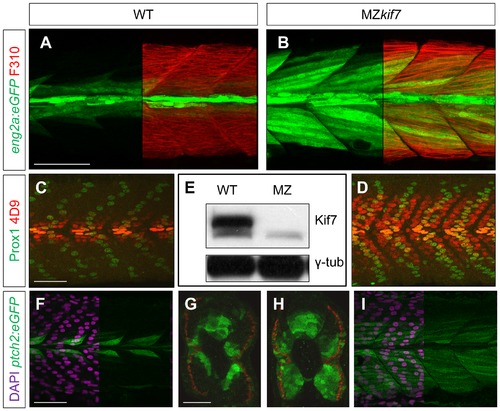
Absence of Kif7 protein and de-repression of Hh target genes in MZkif7 embryos. (A,B) Lateral images of 30 hpf wild-type (A) and MZkif7 mutant (B) embryos expressing eng2a:eGFP (green); note dramatic expansion of eng2a:eGFP expression within the fast-twitch fibers revealed by F310 staining (red) in the merged image (right panel). (C,D) Parasagittal optical sections of wild-type (C) and MZkif7 mutant (D) 30 hpf embryos stained with anti-Prox1 (green) and mAb4D9 (red) (E) Western blot of wild-type (WT) and MZkif7 mutant (MZ) embryo extracts probed with polyclonal rabbit anti-Kif7 antiserum showing complete loss of Kif7 protein (upper band) from MZkif7 embryos. Loading control: γ-tubulin (γ-tub). (F,I) Parasagittal and (G,H) transverse optical sections of 30 hpf wild-type (F,G) and MZkif7 (H,I) embryos expressing a ptch2:eGFP transgene (green) showing the expansion of the ptch2 expression domain in the myotome and neural tube in the absence of Kif7. Nuclei are revealed in left half (of panels F,I) by DAPI staining (purple). The edge of the myotome is shown by mAbF9 (in G,H) marking the superficial slow-twitch muscle fibers. Scale bar: 50 μm. |
|
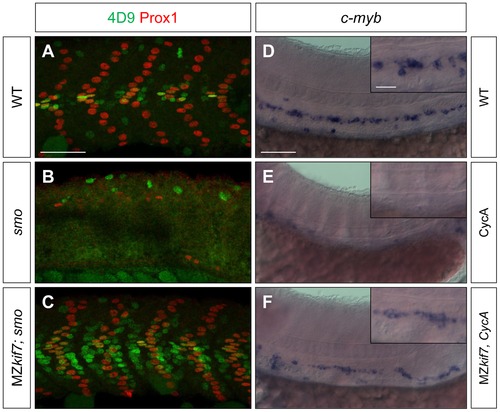
Kif7 acts downstream of Smo to control Hh target genes. (A,B,C) Parasagittal optical sections of 30 hpf wild-type (A), smo mutant (B) and smo;MZkif7 (C) double mutant embryos stained with mAb4D9 (green) and Prox1 (red). Scale bar 50 μm. (D,E,F) Lateral view at the level of the yolk extension of 36 hpf wild-type (D), cyclopamine exposed (E) and MZkif7; cyclopamine (CycA) exposed (F) hybridized with a probe for c-myb RNA, marking hematopoietic stem cells in the ventral floor of the dorsal aorta. Scale bar 50 µm (detail in insets; scale bar 10 μm). |
|
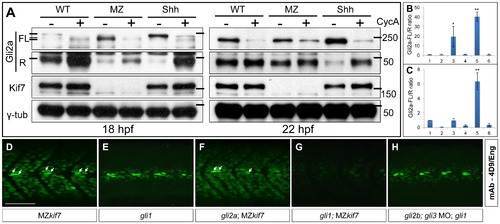
Regulation of Gli processing and activity by Kif7. (A) Western blot analysis showing Gli2a processing in wild-type (WT), MZkif7 (MZ) and Shh mRNA injected (Shh) embryos at 18 hpf (left panel) and at 22 hpf (right panel) compared to the same set treated with cyclopamine (CycA). Full-length (FL) and repressor (R) forms of Gli2a are indicated. The lower panels shows the same blot re-probed with Kif7 and γ-tubulin (loading control) antibodies (B,C) Quantification of the Gli2a FL:R ratio normalized to WT at 18 hpf (B) and 22 hpf (C). Bar graphs numbered 1–6 represent lanes on the blots in (A) from left to right. Error bars represent standard deviation obtained from three independent Western blots including those shown in (A). Single asterisk: P<0.02; double asterisk: P<0.001. (D–H) Parasagittal optical sections of 30 hpf embryos of different genotypes showing expression of Eng-expressing muscle cells as revealed by mAb4D9 (green). The MZkif7 phenotype (D) is largely unaffected by removal of Gli2a (F), other than the loss of the some MP cells (arrows); by contrast, Eng expression is nearly eliminated in the MZkif7;gli1 double mutant embryo (G), despite Gli1 being dispensable for Eng expression in the presence of Kif7 function (E). Knockdown of gli2b and gli3 activity using morpholinos in gli1 mutants (H) has no effect on Eng expression in the myotome. Scale bar: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
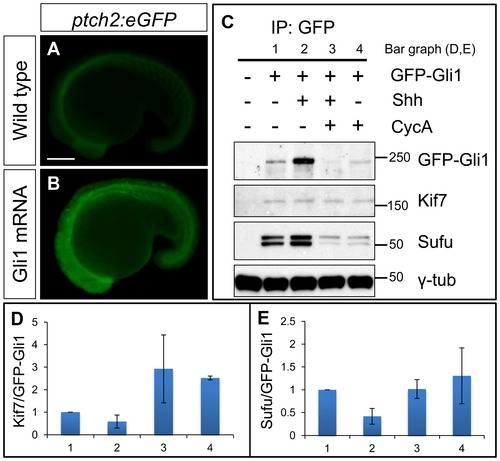
Functional tagged Gli1 associates with Kif7 and Sufu in a Hh dependent manner. (A,B) Lateral views of 20ss ptc2:eGFP embryos uninjected (A) and injected with mCherry-Gli1 mRNA (B) showing the ectopic activation of ptch2:eGFP reporter. Scale bar: 150 μm. (C) Western blot analysis of anti-GFP immune-precipitates from uninjected embryos or embryos injected with a combination of GFP-Gli1 and/or Shh mRNA and/or exposed to cyclopamine (CycA); note the stabilization of GFP-Gli1 in the presence of ectopic Shh and a reduced association between Kif7 and Gli1 (see D for quantification); inhibition of Hh pathway activity by cyclopamine reverses this effect and further enhances the association. The inhibitory association of Sufu and Gli1 is also reduced in response to pathway activation by Shh mRNA injection (see E for quantification). (D) The ratio of Kif7:GFP-Gli1 from experiments described in panel (C); WT (1), Shh mRNA injected (2), CycA exposed and Shh mRNA injected (3), and CycA exposed embryos; showing reduced Kif7-Gli1 association upon pathway activation and increased association when the pathway is inhibited. Error bars represent standard deviation obtained from three independent biological replicates. (E) The ratio of Sufu:GFP-Gli1 from experiments described in panel (C); WT (1), Shh mRNA injected (2), CycA exposed and Shh mRNA injected (3), and CycA exposed embryos; showing reduced Sufu-Gli1 association upon pathway activation and restoration of this association upon pathway inhibition. Error bars represent standard deviation obtained from three independent biological replicates. |
|
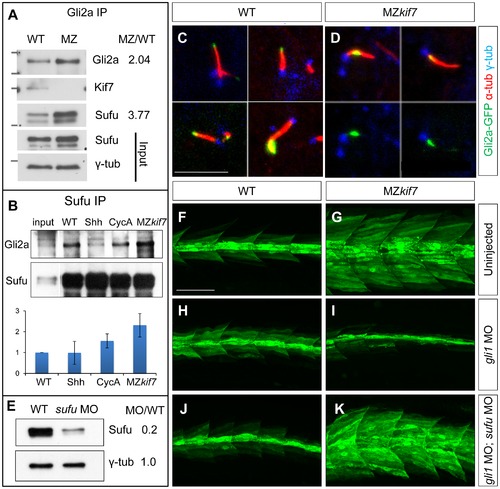
Kif7 modulates Gli2a localization and its association with Sufu. (A) Western blot analysis of anti-Gli2a immune-precipitates of WT and MZkif7 (MZ) embryos, showing association of Kif7 with Gli2a in wild-type embryos and increased association of Sufu with Gli2a in MZkif7 embryos, as indicated by the ratio of the normalized intensities of the MZ to WT signals. (B) Western blot analysis of anti-Sufu immuno-precipitates of WT, Shh RNA injected (Shh), cyclopamine (cycA) exposed and MZkif7 (MZ) embryos; the Gli2aFL:Sufu ratios normalized to wild-type are shown below each lane. Error bars represent standard deviation obtained from three independent biological replicates. Note the increase in levels of Gli2aFL that co-precipitates with Sufu in MZkif7 embryos. (C,D) Localization of a functional eGFP tagged Gli2a protein (green) to primary cilia in wild-type (C) and MZkif7 (D) embryos. The axonemes of the primary cilia are marked by acetylated α-tubulin (red) and the basal bodies by γ-tubulin (blue) staining. In wild-type, Gli2a localizes to the tip of the cilia whereas in MZkif7, localization is restricted to the base of the cilia; the lower two panels in (D) are the same images as in the upper panels but with the red channel removed to show the juxtaposed GFP-Gli2a and γ-tubulin signals more clearly. Scale bar: 5 μm. (E) Western blot of wild-type (WT) and sufu morpholino-injected (sufu MO) embryo extracts probed with anti-Sufu and anti γ-tubulin, showing significant depletion of Sufu levels in the morphants relative to wild-type (MO/WT). (F–K) Parasagittal optical sections of 30 hpf wild-type (WT) or MZkif7 embryos showing the effect on eng2a:eGFP expression of morpholino mediated knockdown of gli1 (H,I) or gli1 and sufu (J,K). Depletion of gli1 in WT embryos (H) has no discernible effect, whereas it causes a drastic suppression of the ectopic expression in MZkif7 (I). This suppression is abrogated by simultaneous removal of Sufu and Gli1 from MZkif7 embryos (K). Scale bar: 50 μm. |
|
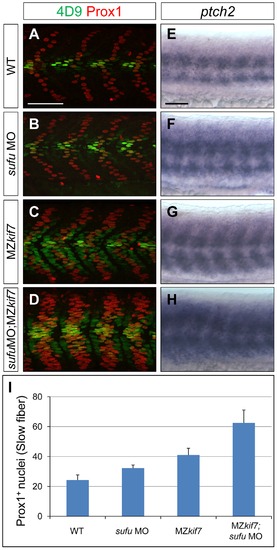
Loss of Sufu further enhances Gli activity in MZkif7 mutants. (A–D) Parasagittal optical sections of 30 hpf WT (A,B) or MZkif7(C,D) embryos stained with anti-Prox1 (red) and 4D9 (green) antibodies; morpholino mediated knock-down of sufu results in significant increase in Prox1 expressing slow twitch muscle cells in WT (B, quantified in I) and a further increase in MZkif7(D) mutants; Scale bar: 50 µm. (E–H) Lateral view of 30 hpf WT (A) or MZkif7(C) embryos hybridized with an antisense probe for ptch2 mRNA; MZkif7 embryo shows an expansion of ptch2 expression; embryos of similar stage and genotype injected with sufu MO result in an enhancement of the levels of ptch2 staining in WT (F) and in MZkif7 (H) embryos; Scale bar: 50 µm. (I) Quantification of Prox1+ slow twitch muscle fiber nuclei per hemisegment in WT, sufu MO injected, MZkif7 and MZkif7 injected with sufu MO. Error bars represent standard deviation obtained from 16–20 hemisegments from >6 embryos. Note the significant increase in MZkif7 compared to WT and a further enhancement in slow fibers by removal of Sufu in MZkif7 mutants. |
|
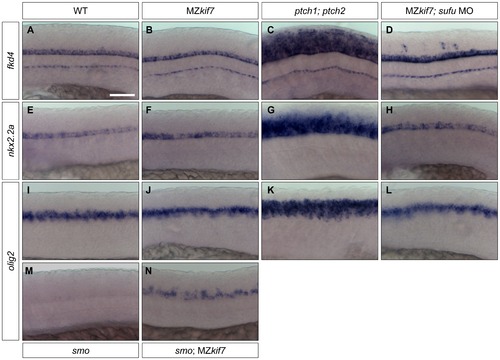
Hh target gene regulation in the neural tube is largely independent of Kif7 and Sufu function. (A–L) Lateral view at the level of yolk extension of 30 hpf WT (A,E,I), MZkif7 (B,F,J), ptch1;ptch2 double mutants (C,G,K) and sufu MO injected MZkif7 mutants (D,H,L) hybridized with antisense probes for fkd4 (A–D), nkx2.2a (E–H) or olig2 (I–L); Note the ventrally restricted domains of fkd4, olig2 and nkx2.2a typical of WT embryos are dramatically expanded in ptch1;ptch2 double mutant embryos (C,G,K) but are unaffected in the absence of Kif7 function. sufu MO injected MZkif7 embryos show wild-type patterns of nkx2.2a or olig2 but ectopically expression fkd4 in scattered cells. (M,N) expression of olig2 is completely lost from the neural tube in smo mutant embryos (M) but is partially restored by removal of kif7 function (N). Scale bar: 50 μm. |
|
Kif7 protein localization is modulated by Hh pathway activity. (A–C) Parasagittal optical sections of the neural tube in 20ss embryos, showing the distribution of the endogenous Kif7 protein. In wild-type (A) and smo (B) embryos, Kif7 accumulates in puncta throughout the cytoplasm as well as in the primary cilium. In ptch1; ptch2 double mutant embryos (C) by contrast, the cytoplasmic puncta are completely absent with Kif7 remaining only at the tips of the cilia. Scale bar: 10 μm. (D–I) similar distributions of Kif7 are seen in the otic vesicle (D–F) and the myotome (G–I) of wild-type and mutant embryos. Insets show a magnified view of a part of each image; note that Kif7 accumulates at the tips of some primary cilia in wild-type (D,G), smo mutant (E,H) but at elevated levels in all cilia in ptch1;ptch2 double mutant (F,I) embryos. Scale bars: 10 μm; Inset scale bar: 1 μm. (J) Quantification of fluorescence intensity of Kif7 from the otic vesicle at 20ss from wild-type (WT), smo mutants and ptch1;2 double mutants, revealing a decrease in Kif7 levels detected by immunofluorescence. Error bars represent standard deviation in spot intensity in pre-processed confocal stacks. (K) Western blot analysis of endogenous Gli2a and Kif7 protein from wild-type (WT), cyclopamine exposed wild-type (WT), Shh RNA injected (Shh) and cyclopamine treated Shh RNA injected (Shh CycA) wild-type embryos exposed to cyclopamine. Note the increase in full-length Gli2a levels following Shh overexpression. Relative levels of Kif7 protein normalized to wild-type are indicated in (L). EXPRESSION / LABELING:
PHENOTYPE:
|
|
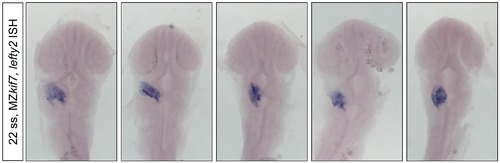
Complete loss of Kif7 does not result in left-right patterning defects. Dorsal view of five 22ss MZkif7 embryos stained with an antisense probe for lefty2 showing correct positioning of the heart tube. Up to 30 MZkif7 embryos were analyzed for lefty2 expression, all of which displayed the correct L-R patterning. |