- Title
-
Gβ1 is required for neutrophil migration in zebrafish
- Authors
- Ke, W., Ye, D., Mersch, K., Xu, H., Chen, S., Lin, F.
- Source
- Full text @ Dev. Biol.
|
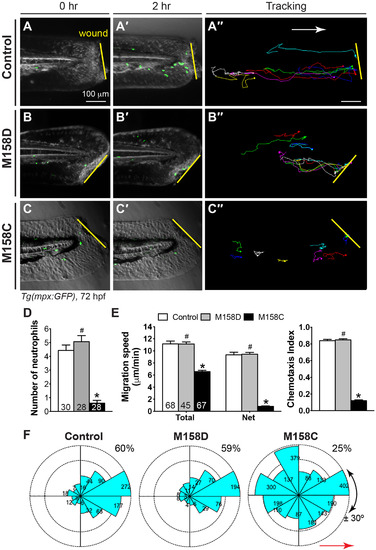
Gβγ signaling is required for wound-induced neutrophil migrationin vivo. 72-hpf Tg(mpx:GFP) embryos pretreated for 30 min with the small-molecule Gβγ inhibitor M158C (25 μM), its inactive compound M158D (25 μM), or 1% DMSO only (control), were wounded (a portion of the tail fin was excised). (A-C′) Snapshots from epifluorescence and bright-field movies of the tail region (right) immediately after wounding (0 h, A-C) and 2 h after wounding (2 h, A′-C′). (A′′-C′′) Representative tracks (multiple colors) delineate routes of migration of individual neutrophils. (D) Number of neutrophils that reach the wound site within 2 h (the number of embryos analyzed is shown). (E) Total and net speeds of migration, and chemotaxis index, of neutrophils in 1% DMSO- (68 neutrophils, 7 embryos), M158D- (45 neutrophils, 5 embryos), and M158C- (67 neutrophils, 9 embryos) treated embryos. (F) Rose diagrams showing the directions of neutrophil movement events relative to the direction of migration (along anteroposterior axis, red line with arrowhead). The direction of migration was analyzed every 2–3 min throughout the time-lapse period, and were grouped into 30° sectors, with the number of events in each sector indicated. The fraction of migration events oriented at an angle of ±30° with respect to the direction of migration is 60% of 750 events in 1% DMSO-treated neutrophils, 59% of 453 events in M158D-treated neutrophils, and 25% of 453 events in M158C-treated neutrophils (p < 0.001, by t-test, two-way ANOVA, for M158C vs DMSO; p < 0.001, by one-way ANOVA for all three groups). Yellow lines: areas of wounding; Red line with arrowhead: the direction of migration. #: p > 0.05 vs control, *: p < 0.001 vs control. EXPRESSION / LABELING:
PHENOTYPE:
|
|
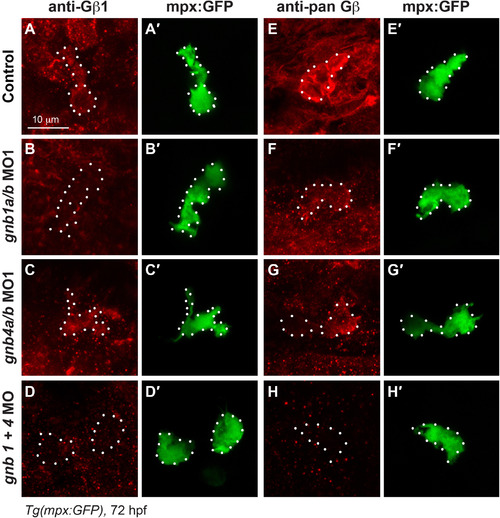
Both Gβ1 and Gβ4 are expressed in neutrophils. (A-H) Confocal images of neutrophils showing the expression of Gβ1 (A-D) and total Gβ (E-H), as detected by immunostaining in 72 hpf-Tg(mpx:GFP) embryos, with or without injection of the indicated MOs. (A′-H′) Corresponding GFP-expressing neutrophils. White dots outline neutrophils. EXPRESSION / LABELING:
PHENOTYPE:
|
|
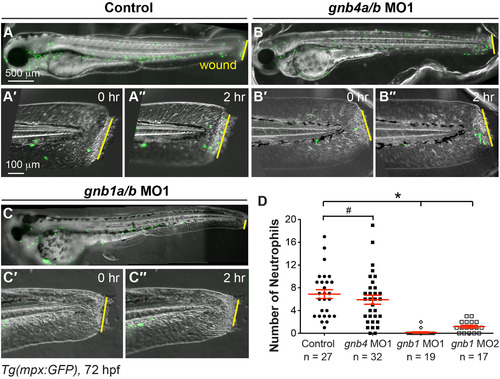
Gβ1, but not Gβ4, is required for wound-induced neutrophil migration. Epifluorescence time-lapse experiments tracking neutrophil migration for 2 h following wounding of the indicated 72 hpf-Tg(mpx:GFP) embryos. (A-C′′) Overlay of epifluorescence and bright-field images of the whole embryo (A-C) and the tail region, immediately after wounding (0 h, A'-C') and 2 h after wounding (2, A′′-C′′). Yellow lines: areas of wounding. (D) Number of neutrophils that reach the wound sites within 2 h of wounding (the number of embryos analyzed is indicated). EXPRESSION / LABELING:
PHENOTYPE:
|
|
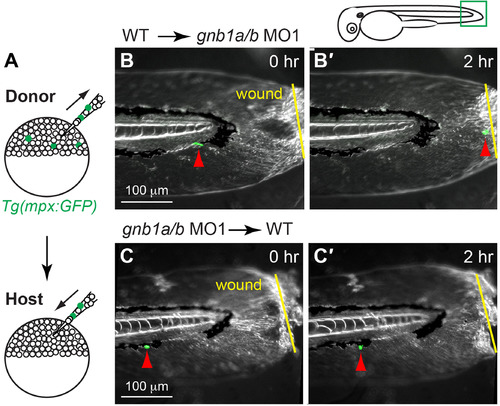
Gβ1 function in neutrophils is required for wound-induced neutrophil migration. (A) Schematic diagram depicting transplantation procedure. Cells from 72 hpf-Tg(mpx:GFP) embryos (donor) were transplanted into wildtype (WT) embryos (host) at the sphere stage. (B-C) Snapshots from epifluorescence and bright-field time-lapse movie (Supplementary Movie 3), showing positions of neutrophils and wound sites immediately (0 h, B, C) and 2 h (2 h, B′, C′) after wounding. (B-B′) gnb1ab MO1-treated host embryos transplanted with cells from control Tg(mpx:GFP) embryo, with wound site (yellow arrows) and nearest GFP-expressing neutrophil (red arrows) indicated. In 6 out of 7 host embryos, transplanted mpx-GFP expressing neutrophils migrated to the wound. (C-C′) Control host embryos transplanted with cells from gnb1a/b MO1-injected Tg(mpx:GFP) embryo, with wound site (yellow line) and nearest GFP-expressing neutrophil (red arrows) indicated. In 6 host embryos, none of the transplanted mpx-GFP expressing neutrophils migrated to the wound. Drawing at top shows wound position and area imaged (green box). |
|
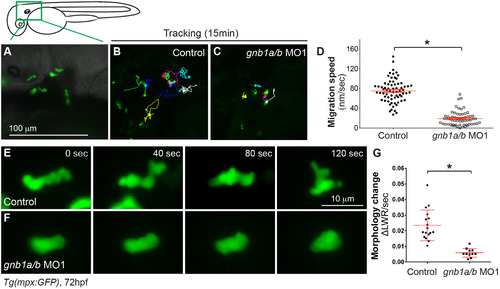
Gβ1 signaling is critical for neutrophil motility. Epifluorescence time-lapse experiments were performed on neutrophils in the head region (green box) of 72 hpf-Tg(mpx:GFP) embryos, using ×5 (A-D) and ×20 (E-G) objectives. (A) Representative image showing GFP-expressing neutrophils in the head region. (B-C) 15-min tracks of neutrophil migration, from time-lapse movies of control (B) and gnb1ab MO1-injected (C) Tg(mpx:GFP) embryos (Supplementary Movie 4). (D) Speed of neutrophil migration in the indicated embryos (69 neutrophils in 6 control embryos, 60 neutrophils in 6 gnb1ab MO1-injected embryos). (E-F) Snapshots from 2-min movies of control (E) and gnb1a/b MO1-injected (F) Tg(mpx:GFP) embryos, showing the morphological changes in neutrophils (Supplementary Movie 5). (G) Changes in length-width ratio (LWR) of neutrophils over time (17 neutrophils in 2 control embryos and 11 neutrophils in 4 gnb1ab MO1-injected embryos were analyzed, *P < 0.0001). EXPRESSION / LABELING:
PHENOTYPE:
|
|
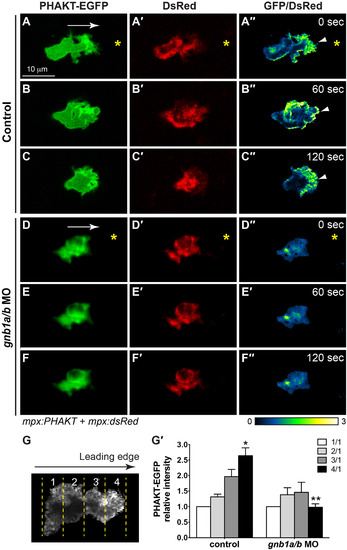
Gβ1 signaling regulates directed neutrophil migration by activating PI3K. (A-F′′) Snapshots from confocal time-lapse imaging (Supplementary Movie 6) of migrating neutrophils expressing PHAKT-EGFP and DsRed in control (A-C) and gnb1a/b MO1-injected (D-F) embryos following wounding of tailfin. (A-F) PHAKT-EGFP. (A′-F′) DsRed. (A′′-F′′) Ratiometric images of PHAKT-EGFP/DsRed. Yellow asterisks: side nearest wound; white arrowheads: PHAKT-GFP enrichment at neutrophil leading front; white arrows: direction of migration. (G-G′) Quantification of ratios of intensity of PHAKT-EGFP/DsRed. (G) Illustration of the method. Neutrophils were selected for “plot profile” analysis using the rectangle tool to span the leading and trailing edges of the cells. The measurements were grouped by quarter and the average GFP/DsRed intensity in each quarter was calculated, with the first quarter representing the trailing region and the fourth quarter representing the leading region. (G′) Graph showing the ratio of intensities of PHAKT-GFP/DsRed in each quarter relative to that in the first quarter in control (5 neutrophils in 5 embryos) and gnb1a/b MO1-injected (7 neutrophils in 7 embryos) embryos. * p = 0.003 vs 1/1 in control, ** p = 0.0001 vs 4/1 in control by t-test, two-way ANOVA; p < 0.0001 among groups in control embryos, by one-way ANOVA; p = 0.587 among groups in gnb1a/b MO1-injected embryos, by one-way ANOVA. |
|
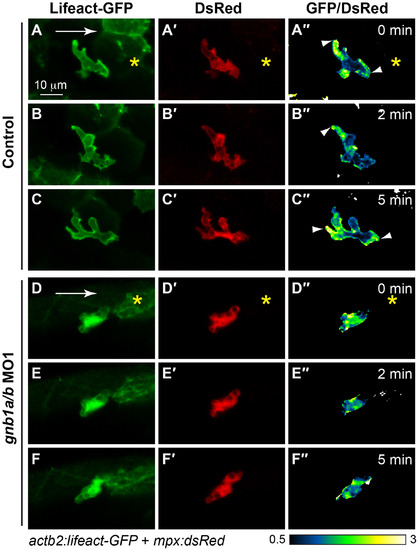
Gβ1 signaling regulates F-actin dynamics in neutrophils. Snapshots from confocal time-lapse imaging (Supplementary Movies 7 and 8) of migrating neutrophils expressing Lifeact-GFP in control and gnb1a/b MO1-injected embryos (3 cells in 3 embryos for each group) following wounding. (A-F) Lifeact-GFP. (A′-F′) DsRed. (A′′-F′′) Ratiometric images of Lifeact-GFP/DsRed. Yellow asterisks: side nearest wound; white arrowheads: sites of Lifeact-GFP enrichment in leading and trailing fronts of neutrophils. EXPRESSION / LABELING:
PHENOTYPE:
|
|
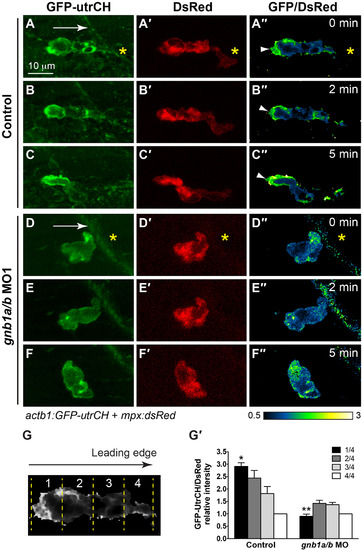
Gβ1 regulates the polarity of stable F-actin accumulation in neutrophils. (A-F) Snapshots from confocal time-lapse imaging (Supplementary Movie 8) of migrating neutrophils expressing GFP-utrCH in control and gnb1a/b MO1-injected embryos following wounding. (A-F) GFP-utrCH. (A′-F′) DsRed. (G′′-L′′) Ratiometric images of GFP-utrCH/DsRed. Yellow asterisks: side nearest wound; white arrowheads: GFP-utrCH enrichment in neutrophil trailing region; white arrows, direction of migration. (G-G′) Quantification of GFP-utrCH/DsRed. (G) Illustration of the method used to quantify ratio of intensities of PHAKT-EGFP/DsRed, as described in Fig. 6G. (G′) Graph showing the ratio of intensities of GFP-utrCH/DsRed in each quarter of the cell relative to that in the fourth quarter (the leading region), in control (5 neutrophils in 5 embryos) and gnb1a/b MO1-injected (9 neutrophils in 4 embryos) embryos. * p = 0.0001 vs 1/4 in control, ** p < 0.0001 vs 1/4 in control, by t-test, two-way ANOVA; p < 0.0001 among groups in control embryos, by one-way ANOVA; p = 0.0003, among groups in gnb1a/b MO1-injected embryos, by one-way ANOVA. EXPRESSION / LABELING:
PHENOTYPE:
|
Reprinted from Developmental Biology, 428(1), Ke, W., Ye, D., Mersch, K., Xu, H., Chen, S., Lin, F., Gβ1 is required for neutrophil migration in zebrafish, 135-147, Copyright (2017) with permission from Elsevier. Full text @ Dev. Biol.