- Title
-
Mutation in utp15 Disrupts Vascular Patterning in a p53-Dependent Manner in Zebrafish Embryos
- Authors
- Mouillesseaux, K., and Chen, J.N.
- Source
- Full text @ PLoS One
|
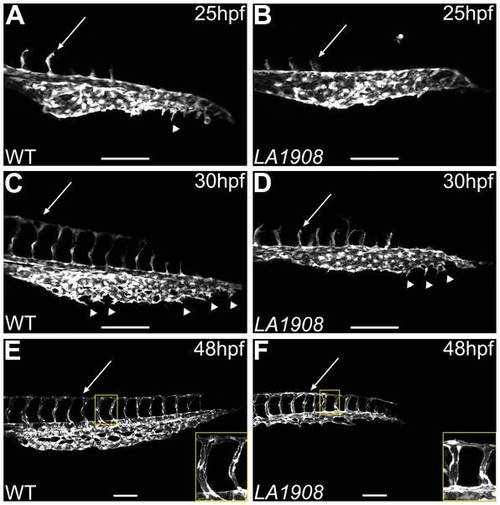
Confocal microscopic analysis of angiogenesis in LA1908 embryos. A–F, Confocal z-stack projection images were captured of the trunk and tail vasculature of wild type (A, C, E) and LA1908 mutant (B, D, F) embryos at 25 (A, B), 30 (C, D), or 48 hpf (E, F). CVP endothelial tip cells are indicated by arrowheads; extent of ISV maturation is indicated by arrows in A–D. Delay of primary ISV sprouting is most prominent at 30 hpf (C and D, arrows), when WT ISVs have reached their dorsal terminus and branched to form the DLAV (C), while mutant ISVs remain stunted (D). By 48 hpf, ISV number is equivalent between WT and mutant embryos and DLAV has formed in both groups (E, F, arrows). Defects in CVP angiogenesis result in a thinner and shorter plexus that lacks the complexity of WT embryos (F versus E). Insets in E and F, enlarged regions indicated by yellow rectangles, showing ISV lumens. Scale bars are 100 μm. |
|
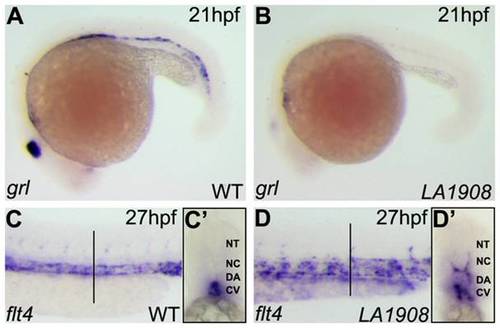
Aberrant expression of arterial and venous endothelial cell markers in LA1908 mutant embryos. A–B, Expression of grl, an arterial EC marker, was dramatically reduced in LA1908 mutant embryos (B versus A) at 21 hpf. C–D, Expression of the venous marker, flt4, was increased and localized ectopically in LA1908 mutant embryos at 27 hpf (D versus C). Vertical black bars, approximate location of vibratome cross-sections shown in C′–D′. C′–D′, Vibratome cross-section of embryos shown in C, D, revealing flt4 expression was appropriately restricted to the CV in 27 hpf wildtype embryos (C′), but is ectopically expressed in the DA and ISVs in LA1908 mutant embryos (D′). Embryos shown are representative from triplicate experiments performed on a minimum of 5 embryos per condition. EXPRESSION / LABELING:
|
|
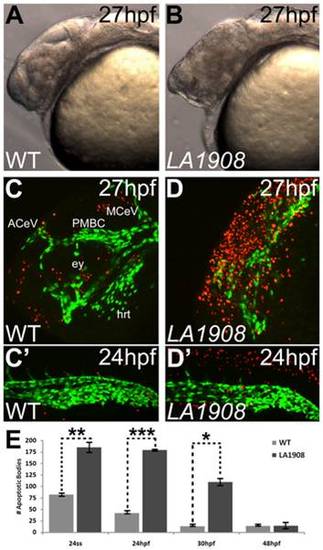
Apoptosis is pervasive in neural tissues of LA1908 mutant embryos. A–B, Brightfield phase-contrast imaging of mutant embryos revealed craniofacial disorganization and extensive cell death (hazy dark brown appearance in midbrain in B versus A). C–D′, Apoptosis is significantly induced in LA1908 mutant embryos (D, D′) relative to stage-matched WT siblings (C, C′). Green fluorescence marks the endothelial cells. Red fluorescence indicates apoptotic cells detected by TUNEL assay. ACeV, Anterior cerebral vein. ey, eye primordium. hrt, embryonic heart. MCeV, Middle cerebral vein. PMBC, Primordial midbrain channel. E, Quantitative and statistical analyses of apoptotic bodies at several stages of development in WT versus mutant embryos. * = p-value<0.05, ** = p-value<0.005, *** = p-value<0.00005 by two-tailed t-test, n = 3 embryos for each condition, data are average±SEM. |
|
The LA1908 locus encodes zebrafish utp15. A, Positional cloning showed tight linkage between LA1908 and utp15. B, Sequence analysis of the utp15 gene revealed an intronic G to A point mutation, abolishing the splice acceptor site preceding exon11 and resulting in three utp15 cryptic splicing variants; a 134 bp deletion (utp15 small), a 26 bp deletion (utp15 medium), and a 36 bp insertion (utp15 large). Inset in (B) shows the relative cDNA sizes of S (utp15 small), W (wild type utp15), M (utp15 medium), and L (utp15 large) variants. C, The protein structure and translational consequences of utp15 splice variants. Utp15 small and medium proteins contain premature stop codons resulting in truncated proteins. Injecting in vitro synthesized mRNA generated from these constructs was unable to rescue LA1908 mutant phenotype. Utp15 large contains a 12 amino acid insertion and displays some ability to rescue phenotype. Red bar, WD40 protein-protein interaction domain. Blue bar, utp15 C-terminal superfamily domain of unknown function. Green bar, altered peptide sequence resulting from cryptic splicing. * = p-value <0.001 by Chi-Squared analysis of Mendelian ratios of a minimum of 100 embryos per condition. Rescue efficiency is calculated as # embryos exhibiting LA1908 mutant phenotype/# total embryos with LA1908 mutant genotype, n = 10 (WT), 28 (small), 11 (medium), and 13 (large) mRNA injected mutant embryos. |
|
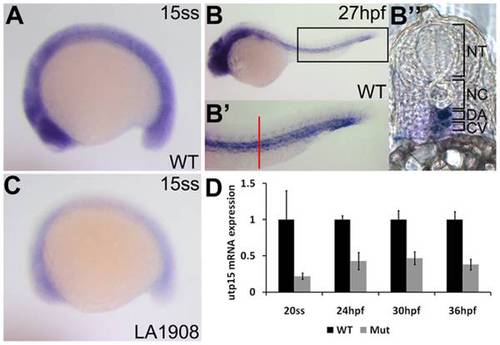
Analysis of endogenous utp15 expression by whole-mount in situ hybridization. A, utp15 is expressed ubiquitously in developing embryos at early stages. B, Expression was restricted to neural and vascular tissues after one day of development. B′, 2x magnification of the rectangular region indicated in (B), showing expression in the dorsal aorta and cardinal vein. Red bar, approximate location of the vibratome cross-section shown in (B′′). C–D, utp15 was dramatically reduced in mutant embryos, as shown by in situ hybridization (C) and qRT-PCR (D) analyses. A–C, Embryos shown are representative of triplicate experiments performed on a minimum of 5 embryos per condition. D, Data shown are the average fold changes in expression±SEM from two replicate qRT-PCR assays using pooled RNA from 20 embryos per condition. EXPRESSION / LABELING:
|
|
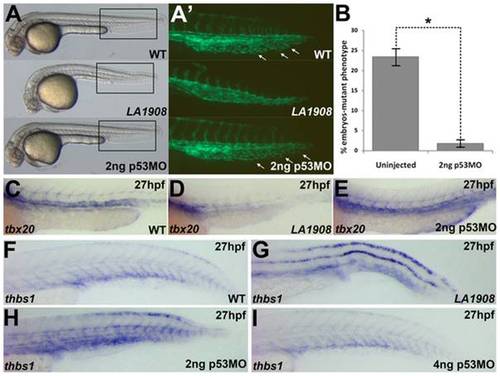
Loss of function of utp15 induces p53 activity. A, Brightfield Phase contrast images of 30 hpf embryos. LA1908 mutant embryos manifest defects in the brain and the vasculature as well as a significantly shorter body when compared to their wild type siblings. Injection of 2 ng p53MO is sufficient to prevent CNS necrosis (A), restore normal CVP morphology (A′), and normalize the gross morphology of the mutant embryos. A′, Magnified image of boxed regions of Tg[kdrl:GFP] embryos in (A). Note normal ISV patterning and presence of EC sprouting within the CVP (white arrows), resulting in a prototypical plexus. B, Chi-squared analysis of WT:Mutant ratios from 3 independently replicated experiments. n = 220 uninjected control embryos, 298 2 ng p53MO-injected embryos, * = p-value<0.001. Data are average +/- SEM. C–E, Analysis of tbx20 expression, a marker of arterial endothelial cells, demonstrated p53MO also restores normal expression levels and patterning (E) which were lost in mutants (D) relative to WT (C) embryos. Embryos are representative of experiments performed in triplicate on a minimum of 5 embryos per condition. F–H, thbs1 is expressed in a p53-dependent manner. Under normal conditions (F), expression is observed in the floor plate, dorsal neural tube, and in the ventral somites/axial vasculature. In mutant embryos, thbs1 expression is substantially upregulated (G). Knockdown of p53 reduced thbs1 expression in a dose-dependent manner. Roughly half (18/41) of the embryos injected with 2 ng p53MO have thbs1 levels as shown in (H), with the remainder (23/41) displaying WT (F) levels. When the dose is increased to 4 ng (I), 82% of embryos (31/38) had WT (F) levels of thbs1 mRNA, while only 18% (7/38) appeared as in (H). |
|
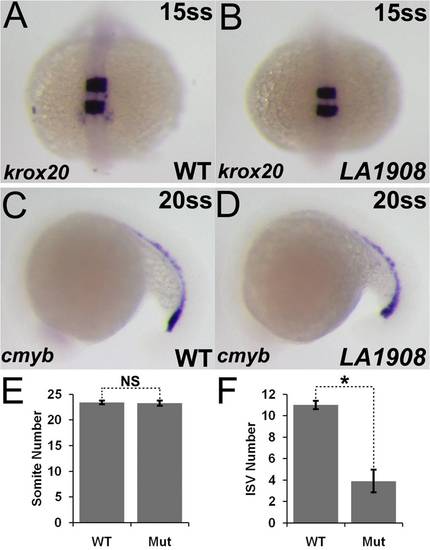
Gene expression in LA1908 is not globally disrupted. A–D, Markers of neural (krox20, A, B) and hematopoietic (cmyb, C, D) differentiation were indistinguishable between wild type and mutant embryos. n = 20 embryos for each condition. E–F, Development was not generally delayed, as somite number was not significantly different between LA1908 mutant and wild type siblings (E). Rather, delay was specific to vascular tissues, exemplified by decreased ISV number in mutant versus wild type embryos at 21 hpf (F). Ten embryos were analyzed per condition. NS = not significantly different, * = p<0.00005. EXPRESSION / LABELING:
PHENOTYPE:
|
|
ISVs of both arterial and venous origin are present in LA1908 mutant embryos. A–B, Confocal z-stack projection images of Tg[kdrl:GFP]LA1908 wildtype (A) or mutant (B) embryos. A′–B′′, Single 2.75 μm z-slices of the region outlined in yellow in (A, B), revealing the axial vessel origin of ISVs in day 2 embryos. A′–B′, ISVs originating from the DA are indicated by a red "A". A′′–B′′, ISVs originating from the PCV are indicated by a blue ′′V′′. Scale bars are 100 μm. |
|
Expression of growth factors is unchanged in LA1908 mutant embryos. Expression of bmp2b (A–D), bmp4 (E–H), and vegfaa (I–L) is indistinguishable between stage-matched wild type (A, C, E, G, I, K) and mutant (B, D, F, H, J, L) embryos. Furthermore, expression of vegfaa, the pro-angiogenic signal for ISV angiogenesis, persists in the ventral somites through the time-point after which apoptosis has resolved in LA1908 mutant embryos (K, L), whereas putative CVP pro-angiogenic signals in the ventral tail, bmp2b and bmp4, are absent from the ventral tail after one day of development (C, D, G, H), possibly explaining the differential ability of CVP versus ISV angiogenesis to recover after initial delay. EXPRESSION / LABELING:
|
|
Induction of p53 mRNA expression in LA1908 mutant embryos. A–B, Expression of p53 mRNA is strongly induced by loss of utp15. Upregulation is observed in all tissues in mutant (B) relative to wildtype (A) embryos. C–D, Vibratome cross-section of embryos shown in Figure 6 F–G, revealing induction of thbs1, particularly in the dorsolateral roof and floor plate of the neural tube. EXPRESSION / LABELING:
|