- Title
-
Endoderm-derived Sonic hedgehog and mesoderm Hand2 expression are required for enteric nervous system development in zebrafish
- Authors
- Reichenbach, B., Delalande, J.M., Kolmogorova, E., Prier, A., Nguyen, T., Smith, C.M., Holzschuh, J., and Shepherd, I.T.
- Source
- Full text @ Dev. Biol.
|
Endoderm and mesodermal hand2 function are required for normal ENS and intestinal smooth muscle development but hand2 is not required for the initial migration of vagal neural crest to the anterior end of the intestine and endoderm is not required for mesodermal hand 2 expression. (A, D, G, J, M, O) wild-type embryos, (B, E, H, K, N, P) cas/sox32 morphant embryos and (C, F, I, L) hand2 morphants. (A–C) Ventral view of the vagal region of 36 hpf embryos that have been hybridized with riboprobes for crestin showing a failure of vagal NCC migration to the anterior end of the intestine in sox32 morphants but no affect on these NCC in hand2 morphants. (D–F) Lateral view of 60 hpf embryos that have been hybridized with riboprobes for phox2b. (G–I) Close up lateral view of the intestine of 60 hpf embryos that have been hybridized with riboprobes for phox2b showing a failure of phox2b expressing cells to populate the entire length of the intestine in cas/sox32 and hand2 morphants. (J–L) Close up lateral view of the intestine of 60 hpf embryos that have been hybridized with riboprobes for myh11 showing a reduction/loss of myh11 expressing intestinal smooth muscle cells in the intestine of cas/sox32 and hand2 morphants. (M, N) Lateral view of the intestine of 72 hpf embryos wholemount in situ hybridized embryos that have been hybridized with riboprobes for α SMA. Black boxes in panels D–F are the regions that are shown in close up in panels G–I. Arrowheads (M) indicate the αSMA expressing cells in the intestine. (O, P) Ventral view of 30 hpf wholemount in situ hybridized embryos that have been hybridized with an antisense probe for hand2. Arrows (A, C) indicate the migrating enteric precursors. White arrowheads (G, I) indicate phox2b expressing cells in the intestine. Black arrowheads (J, L) indicate myh11 expressing cells in the intestine. Black arrowheads (M) indicate the αSMA expressing cells in the intestine Anterior is to the left. EXPRESSION / LABELING:
|
|
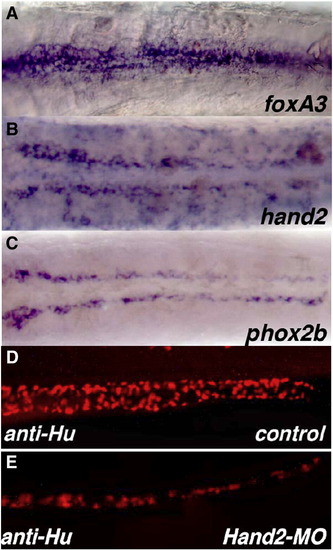
Comparison of the expression of hand2 to endodermal marker foxA3 and ENS precursors marker phox2b and the reduction of ENS neurons in hand2 morphants. (A–C) Ventral views of the intestine of 60 hpf wholemount in situ hybridized embryos that have been in situ hybridized with riboprobes for foxA3 (A), hand2 (B) and phox2b (C). (D, E) Lateral views of the intestine of 96 hpf wild type (D) and hand2 morphant (E) embryos that have been stained with an anti-Hu antibody showing that there is a reduction in the overall neuron number in hand2 morphants as compared to wild type. (A–C) Yolk has been removed from the embryos. Anterior is to the left. |
|
The expression pattern of shh and ptc-1 correlate with the development of ENS. (A, B, E, F) Wholemount embryos hybridized in situ with a shh antisense probe at the indicated developmental stages. (C, D, H) Wholemount embryos hybridized in situ with a ptc-1 antisense probe at the indicated developmental stages. (G) 60 hpf wholemount in situ hybridized embryos hybridized with a phox2b antisense probe to reveal the distribution of the ENS NCC in the intestine. (I) Cross-section taken through a 60 hpf embryo hybridized with a phox2b antisense probe to reveal the distribution of ENS NCC in the intestine. (J) Cross-section taken through a 60 hpf embryo hybridized with a ptc-1 antisense probe. At all stages examined, shh and ptc-1 are expressed in regions that correlate with the development of the ENS. Furthermore, comparison of the pattern of ptc-1 expression in the intestine at 60 hpf shows that phox2b expressing ENS NCC are located in the ptc-1 expression domain. (K–K″) Comparison of the pattern of ptc-1 expression (K′, green channel) to that of phox2b (K, red channel) in the intestine at 42 hpf by double fluorescent in situ hybridization shows that phox2b expressing ENS NCC co-express ptc-1 (K″, merge channels). Expression was documented by confocal stacks of images (L–L″). Ventral view of the vagal region of a 36 hpf wildtype embryo showing a comparison of the pattern of ptc-1 expression (L′, green channel) to that of phox2b (K, red channel) by double fluorescent in situ hybridization showing pre-enteric ENS NCC's express ptc-1 (K″, merge channels). (A) Lateral view of embryo. (B, D, F, G, H, K–K″, L–L″) Ventral views of embryos with the yolk removed. (C, E) Dorsal views of embryo. not (A, B, C, E, F) indicates notochord. end (A–C) indicate endoderm. * (D, G, H, I, J) indicates the gut lumen. Arrows (K–K″) indicate ptc-1 phox2b coexpressing cells. The cells delineated by the white line in (L, L″) are the migrating pre-enteric ENS NCC. Scale bar (K″, L″) is 20 μm. In all wholemounts (A–H) anterior is to the left. |
|
Hedgehog pathway mutants lack enteric ganglia. (A–I) Dorsal views, anterior to the left. Enteric ganglia colonizing the gut express phox2b in wild type (A, black arrows) while syu-/- (B) and smu-/- (C) embryos show no phox2b expression around the gut tube. (D) Expression of ret in 36 hpf wild type embryos in migrating enteric precursors (black arrows). In syu-/- (E) and smu-/- (F) no ret expressing migrating cells can be detected. Wild type embryos at 36 hpf show crestin expression in vagal neural crest cells migrating to the anterior gut (G, black arrows). syu-/- (H) and smu-/- (I) embryos lack crestin expressing vagal neural crest cells. |
|
Cyclopamine treatment causes a failure of enteric precursors to populate the entire length of the intestine and to differentiate into enteric neurons and also results in a failure of intestinal smooth muscle differentiation. (A, D, G, J) Wildtype control embryos, (B, E, H, K) 24–26 hpf cyclopamine treated embryos (C, F, I, L) 36–60 hpf cyclopamine treated embryos. (A–C) Lateral view of 60 hpf wholemount embryos hybridized in situ with a phox2b antisense probe to reveal the distribution of ENS NNC precursors in the intestine. Treatment with cyclopamine results in a significant reduction/absence of ENS precursors along the length of intestine. (D–E) Lateral view of 60 hpf wholemount in situ hybridized embryos hybridized with a myh11 antisense probe. Treatment with cyclopamine results in a loss of this early smooth muscle marker expression in the intestine. (G–I) Lateral view of 72 hpf wholemount in situ hybridized embryos hybridized with a α SMA antisense probe. Treatment with cyclopamine results in a loss of this late smooth muscle marker expression in the intestine. (J–L) Lateral views of 96 hpf HuC-gfp embryos stained with anti-GFP antibody to shows a significant reduction in the number of enteric neurons in the intestine of cyclopamine treated embryos. Treatment conditions are indicated in lower right corner of each panel. Black arrowheads (B, C) indicate the few phox2b expressing cells in the intestine of cyclopamine treated embryos. Arrow (E) indicates pharyngeal arch myh11 expression. White arrowheads (L) indicate differentiated neurons in the intestine. Anterior is to the left. |
|
ENS NCC fail to migrate from the vagal region to the anterior end of the intestine in 24–36 hpf cyclopamine treated embryos. (A, B) Ventral view of the anterior end of the intestine in 36 hpf wildtype (A) and 24–36 hpf cyclopamine treated (B) embryos that have been hybridized with an antisense probe to crestin. Arrows indicate migrating ENS NCC. Anterior is to the left. EXPRESSION / LABELING:
|
|
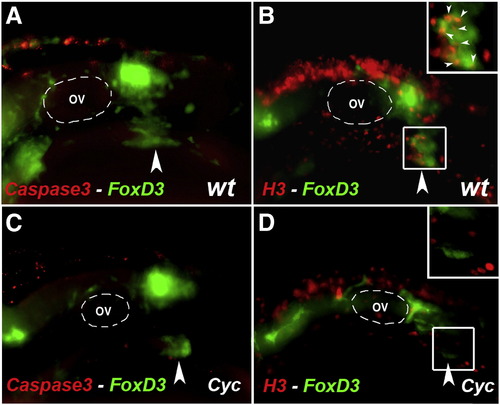
Cyclopamine treatment causes no change in apoptosis but a decrease proliferation in the vagal NCC and ENS NCC. (A–D) Lateral views of the vagal region of 30 hpf wildtype (A, B) and 24–30 hpf cyclopamine treated (C, D) Foxd3:GFP transgenic embryos. Embryos were immunocytochemically double stained with anti gfp antibody (green) (A–D) to reveal the distribution of vagal NCC and ENS NCC's and anti activated caspase 3 antibody (red) (A, C) to reveal apoptotic cells and anti-phosphohistone H3 (red) (B, D) to reveal proliferating cells. OV (A–D) indicates otic vesicle. Large arrowheads (A–D) indicate the stream of vagal NCC that gives rise to ENS. White boxes (B, D) indicate the region that is shown in close up in the insert. Small white arrowheads (B) indicate proliferating cells. Anterior is to the left. EXPRESSION / LABELING:
|
|
shh over-expression leads to an increase in enteric neurons. Dorsal (A, B) and lateral (C, D) views of phox2b in enteric ganglia of 72 hpf control (A, C) and shh injected embryos (B, D). (E) Bar graph summarizes the results from one of three independent shh injection experiments. Enteric ganglia from shh injected embryos contained more phox2b expressing cells around the gut (P = 2.7e - 05; Student's t-test). The error bars represent the standard deviation. Arrows and arrowheads (A–D) indicate phox2b expressing ENS precursors. EXPRESSION / LABELING:
|
|
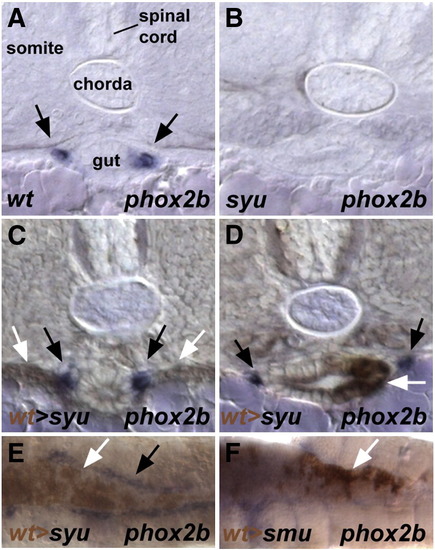
Transplanted wild type endoderm rescues the enteric ganglia in syu mutants. (A–D) Cross-sections taken through 72 hpf embryos. (E, F) ventral views with anterior to the left of 72 hpf embryos. In wild type embryos phox2b expressing enteric neurons are located on the lateral edge of the gut tube (A). No phox2b expressing cells are found around the gut of syu mutant embryos (B). Transplanted sox32 injected cells are recruited to the endoderm of syu mutants and rescue the enteric ganglia formation (C–E). Endodermal transplants from sox32 injected wild type embryos are not able to rescue enteric ganglia formation in smu mutants (F). Black arrows indicate to phox2b expressing enteric neurons (A, C, D, E); white arrows point to transplanted endoderm (C–F). EXPRESSION / LABELING:
|
|
Expression of gdnf in the intestinal mesenchyme requires endoderm and HH signaling but not hand2 function. (A, F, K) wildtype control embryos, (B, G, L) hand2 morphants (C, H, M) cas/sox32 morphant embryos, (D, I, N) 24–26 hpf cyclopamine treated embryos and (E, J, O) 36–60 hpf cyclopamine treated embryos. (A–E) Ventral views of 36 hpf wholemount embryos that have been hybridized with a gdnf antisense probe and the yolk removed. In sox32 morphant (C) and 24–36 hpf cyclopamine treated embryos (D) there is a loss of gdnf expression at the anterior end of the intestine. (F–J) Lateral view of 60 hpf wholemount embryos, that have been hybridized with a gdnf antisense probe, showing the loss of intestinal gdnf expression in sox32 morphants and cyclopamine treated embryos. (K–O) Lateral view of the intestine of 60 hpf wholemount embryos that have been hybridized with a gdnf antisense probe. Arrows (A, B, E) indicate gdnf expression at the anterior end of the intestine. Arrow heads (K, L) indicate the gdnf expression along the length of the intestine. Anterior is to the left. |
Reprinted from Developmental Biology, 318(1), Reichenbach, B., Delalande, J.M., Kolmogorova, E., Prier, A., Nguyen, T., Smith, C.M., Holzschuh, J., and Shepherd, I.T., Endoderm-derived Sonic hedgehog and mesoderm Hand2 expression are required for enteric nervous system development in zebrafish, 52-64, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.