- Title
-
Deficiency of the HGF/Met pathway leads to thyroid dysgenesis by impeding late thyroid expansion
- Authors
- Fang, Y., Wan, J.P., Wang, Z., Song, S.Y., Zhang, C.X., Yang, L., Zhang, Q.Y., Yan, C.Y., Wu, F.Y., Lu, S.Y., Sun, F., Han, B., Zhao, S.X., Dong, M., Song, H.D.
- Source
- Full text @ Nat. Commun.
|
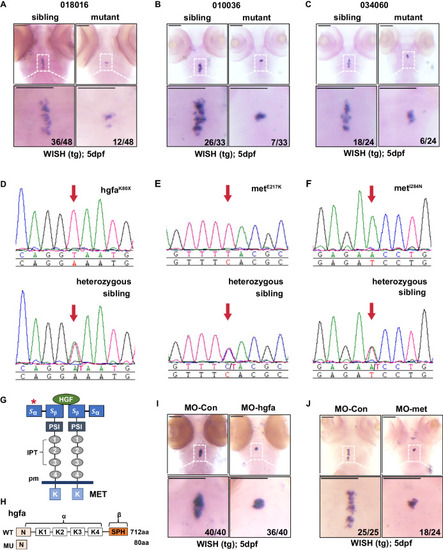
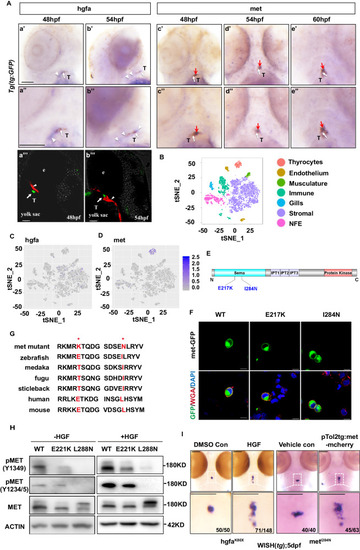
Identification of hgfa and met mutations from three zebrafish lines with abnormal thyroid morphology by whole-exome sequencing and positional cloning. |
|
The orthologous human MET mutations, I284N and E217K, impair HGF signaling. |
|
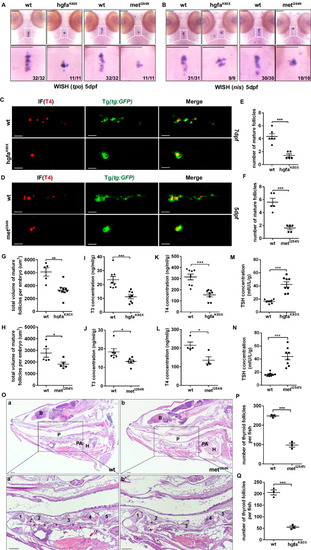
The hgfaK80X and metI284N mutations result in hypothyroidism in zebrafish. |
|
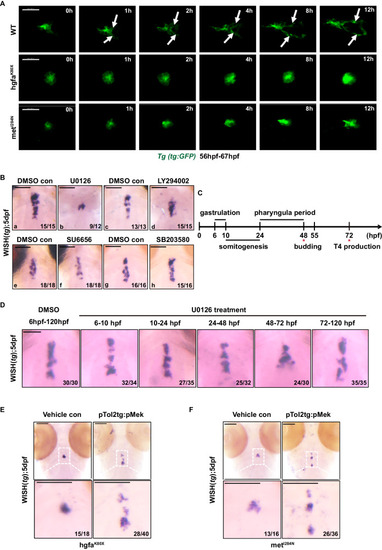
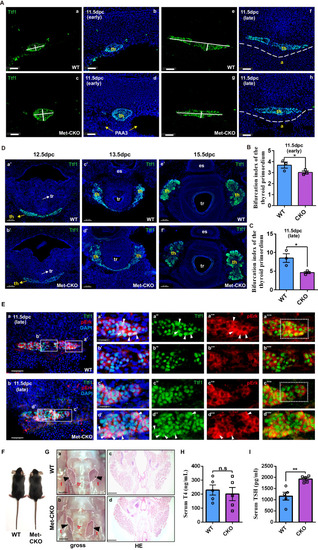
Hgf/met promotes caudal expansion along the pharyngeal midline during the late stage of thyroid development via the MAPK signaling pathway. |
|
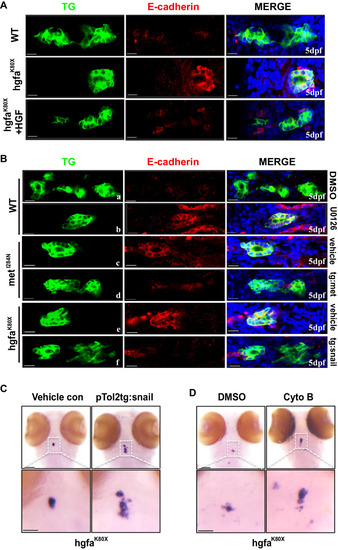
Hgf/met downregulates E-cadherin by activating MAPK-snail in vivo. |
|
HGF-MET-ERK signaling promotes the thyroid bifurcation in mice. |