- Title
-
Glypican 4 and Mmp14 interact in regulating the migration of anterior endodermal cells by limiting extracellular matrix deposition
- Authors
- Hu, B., Gao, Y., Davies, L., Woo, S., Topczewski, J., Jessen, J.R., Lin, F.
- Source
- Full text @ Development
|
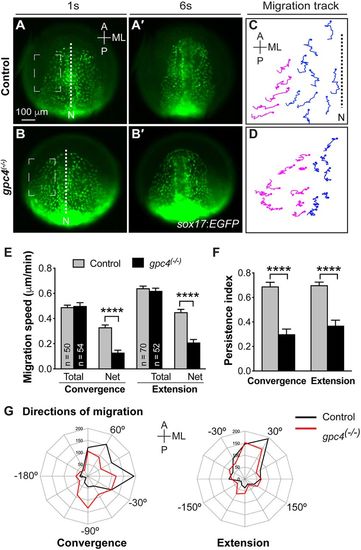
Glypican 4 is required for efficient convergence and extension movements of cells of the anterior endoderm during early segmentation. Epifluorescence time-lapse experiments performed on the anterior endoderm of one- to six-somite stage (s) Tg(sox17:EGFP) embryos (Movie 1). (A-B′) Representative still images from movies at 1s (A,B) and 6s (A′,B′). Dashed squares indicate locations in which cell migration was analyzed. (C,D) Representative migration tracks of two populations of endodermal cells detected in A and in B. Blue and magenta tracks represent cells that migrate primarily in the anterior and medial directions, respectively. Solid circles indicate the endpoint of migration. N, notochord. (E-G) Characteristics of migration. Six embryos of each genotype were analyzed, and the number of cells analyzed for each genotype is indicated in the graph. (E) Total and net speeds of convergence and extension. (F) Persistence index for migration. ****P<0.0001; Student's t-test. (G) Direction of cell migration during the time-lapse period (5 min intervals, grouped into 30° sectors). A, anterior; P, posterior; ML, medial-lateral. EXPRESSION / LABELING:
PHENOTYPE:
|
|
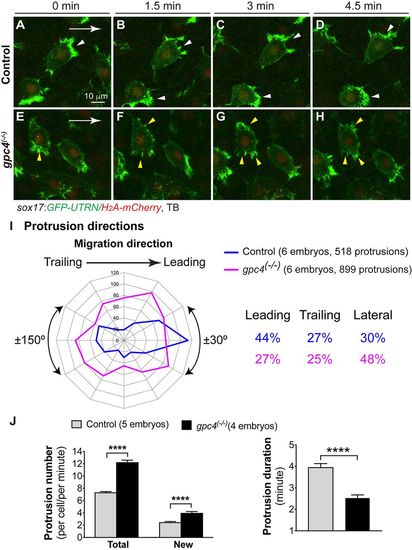
Glypican 4 is required to maintain polarized actin-rich protrusions on migrating endodermal cells. Actin dynamics were assessed by tracking endodermal cells expressing GFP-UTRN (Movie 2). (A-H) Snapshots from confocal time-lapse imaging at different time points. Broader lamellipodia are marked by white arrowheads (control cells) and smaller lamellipodia by yellow arrowheads (gpc4 mutant cells). White arrows indicate the direction of migration of endodermal cells. (I) Direction of protrusions relative to the direction of cell migration in sibling and gpc4 mutant embryos (2 min intervals, grouped into 30° sectors). Percentage of protrusions in various directions (leading, ±30°; trailing, ±150°; lateral, ±30-150°) is shown. (J) Average total protrusions (in each endodermal cell, as assessed at 1 min intervals throughout the imaging period, 1286 protrusions in 11 control cells, 1091 protrusions in eight mutant cells), newly formed protrusions (in each endodermal cell per minute, 443 protrusions in 11 control cells, 326 protrusions in six mutant cells) and the duration of protrusions in control (50 protrusions, 11 cells) and gpc4 mutants (31 protrusions, eight cells). ****P<0.0001; Student's t-test. |
|
Glypican 4 regulates Rac1 activity in migratory endodermal cells. (A) Schematic diagram illustrating the endoderm transplantation procedure. Donor embryos obtained from crossing gpc4 heterozygous fish were injected with sox32 and PDB-GFP RNAs plus rhodamine-dextran (a marker of cell volume) at the one-cell stage. At the blastula stage, 30-50 donor cells were transplanted into host embryos obtained from crossing gpc4 heterozygous fish. (B-C″) Confocal time-lapse analysis of endodermal cells expressing (B,C) PDB-GFP, a fluorescent Rac1 probe, and (B′,C′) dextran. Rac activity was determined as the ratio of the PDB-GFP:dextran signals, and is displayed as radiometric pseudocolored images (B″,C″). Yellow indicates a higher value of PBD relative to dextran. White arrows indicate the direction of migration of endodermal cells (Movie 3). (D) The mean ratio of PDB-GFP:dextran in indicated embryos. The numbers of embryos and cells analyzed are shown. ****P<0.0001; Student's t-test. |
|
Glypican 4 regulates endodermal migration in a non-cell-autonomous manner. Epifluorescence time-lapse experiments performed on the anterior endoderm of Tg(sox17:EGFP) host embryos transplanted with rhodamine-labeled donor cells (magenta). (A-C) gpc4 mutant donor cells transplanted into wild-type Tg(sox17:EGFP) hosts. (D-F) Wild-type donor cells transplanted into gpc4 mutant hosts (Movies 4 and 5). (A,A′,D,D′) Representative still images of anterior endoderm from movies. N, notochord; A, anterior; P, posterior; ML, medial-lateral. (B,C,E,F) Representative tracks delineate routes of migration of donor (B,E, magenta) and host (C,F, green) endodermal cells. (G,H) Total and net speeds of convergence and extension movements by donor (magenta) and host (green) endodermal cells. n=3 embryos per group. The number of endodermal cells tracked is indicated in the graph. #P>0.05; Student's t-test. |
|
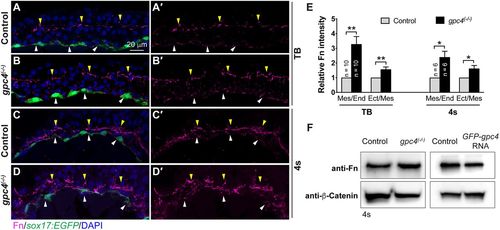
Fibronectin (Fn) expression is increased in gpc4 mutant embryos and reduced in GFP-Gpc4 expressing embryos. (A-E) Immunostaining of transverse cryosections for Fn deposition. (A-D′) Representative confocal z-stack images showing Fn (magenta) and nuclei (DAPI, blue) in embryos indicated. Fn assembly at mes/end (white arrowheads) and ect/mes (yellow arrowheads) boundaries. (E) Relative Fn intensity at mes/end and ect/mes boundaries in control and gpc4 mutant embryos. The number of embryos analyzed is shown in the graph. (F) Western blot showing the expression of Fn and β-catenin (internal control) in embryos indicated. **P<0.01, *P<0.05; Student's t-test. |
|
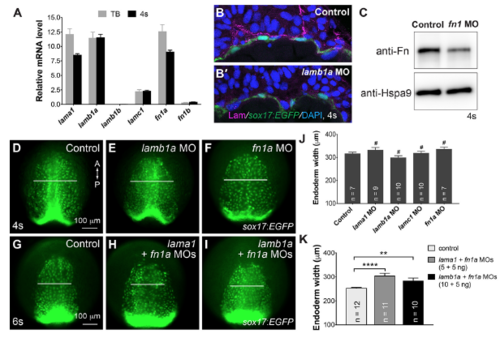
Endodermal defects in gpc4 mutants are suppressed by knocking down fn1a and lamb1a. (A-D) Epifluorescence still images of the anterior region of the endodermal sheet in 6s embryos derived from crossing of gpc4/Tg(sox17:EGFP) heterozygous fish injected with or without MOs targeting fn1a and lamb1a (5 ng each). Anterior-dorsal view. White lines of equivalent length indicate width of the anterior endodermal sheets. A, anterior; P, posterior. (E) Average width of anterior endodermal sheet in embryos shown in A-D. Number of embryos analyzed in each group is indicated. *P<0.05, **P<0.01, ****P<0.0001; Student's t-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
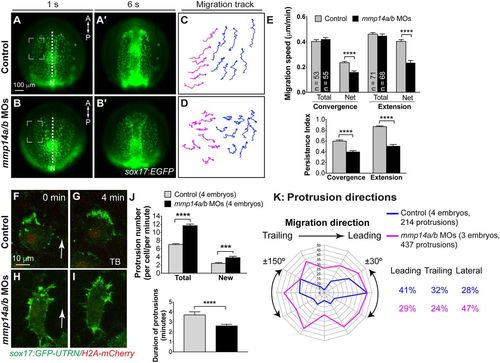
Mmp14a and Mmp14b are required for convergence and extension movements of the anterior endodermal cells. (A-E) Epifluorescence time-lapse experiments for indicated embryos (Movie 6). (A-B′) Still images from movies at 1s and 6s. Dashed squares denote regions in which cells were analyzed. A, anterior; P, posterior. (C,D) Representative migration tracks of anterior endodermal cells in A and B. Blue and magenta tracks represent cells that migrated primarily in the anterior and medial directions, respectively. (E) Total and net speeds of convergence and extension movements, persistence index of cell migration, for the entire lengths of movies, in the embryos indicated (five embryos per group). The numbers of cells analyzed are indicated in the graph. ****P<0.0001; Student's t-test. (F-K) Actin dynamics as assessed by confocal time-lapse imaging of anterior endodermal cells expressing GFP-UTRN in the embryos indicated. (F-I) Representative confocal still images at 0 and 4 min (Movie 7). Arrows indicate direction of migration. (J) Total number (in each endodermal cell at 1 min intervals for the imaging period, 671 protrusions from six control cells, 926 protrusions from six morphant cells), newly formed protrusions (in each endodermal cell per minute, 198 protrusions in six control cells and 280 protrusions in six morphant cells) and duration of protrusions (35 protrusions from six control cells, 34 protrusions from six morphant cells). ***P<0.001; ****P<0.0001; Student's t-test. (K) Direction of protrusions relative to the direction of cell migration in sibling and mmp14a/b MOs-injected embryos (2 min intervals, grouped into 30° sectors). Percentage of protrusions in different directions (leading, ±30°; trailing, ±150°; lateral, ±30-150°) is shown. EXPRESSION / LABELING:
PHENOTYPE:
|
|
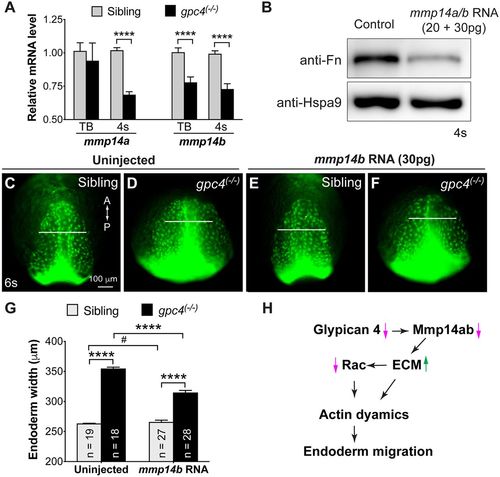
Gpc4, Mmp14a and Mmp14b interact to regulate endodermal migration. (A) Relative mRNA levels of mmp14a and mmp14b when compared with eef1a in embryos indicated, as determined by qRT-PCR. ****P<0.0001, Student's t-test. (B) Western blot showing the expression levels of Fn and heat-shock protein 9 (Hspa9, internal control) in control and mmp14a/b RNA-injected embryos. (C-F) Epifluorescence still images of the anterior region of the endodermal sheet in 6s embryos derived from crossing gpc4/Tg(sox17:EGFP) heterozygous fish injected with or without mmp14b RNA. White lines of equivalent length indicate the width of the anterior endodermal sheet. A, anterior; P, posterior. (G) Average width of anterior endoderm. The numbers of embryos analyzed is indicated. ****P<0.0001, #P>0.05; Student's t-test. (H) Proposed model for how Gpc4 regulates the migration of endodermal cells at early segmentation. Magenta arrows indicate decreases in expression; green arrow indicates increase in expression. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The expression of gpc4 and vangl2 during gastrulation and early segmentation. (A) Expression of gpc4, vangl2 and vangl1 relative to that of foxa2, and endoderm marker, as determined by qRT-PCR, in GFP+ cells sorted from Tg(sox17:EGFP) embryos at 18s. Bars represent the mean±s.e.m. (B-I′′) The expression of gpc4 and vangl2 transcripts in Tg(sox17:EGFP) embryos at 80%E and 10 s, as detected by WISH. (B, D, F, H) Images of the whole embryo. White lines indicate the cross-sectional plane. (C-C′′, E-E′′, G-G′′, I-I′′) Transverse sections of the embryos. (C, E, G, I) Overlays of anti-GFP immunofluorescence staining (sox17:EGFP panels) and ISH for vangl2 and gpc4 (ISH panels), in endodermal cells (red arrowheads). |
|
Gpc4, but not Vangl2, is required for convergence of the anterior endoderm. (A-F) Expression of foxa2 (A-C) and sox17 (D-F) in the indicated embryos, as detected by WISH. Lateral (A-C, D-F) and anterior-dorsal (A-C) views. Yellow lines of equivalent length indicate width of the anterior endodermal sheets. Red lines of equivalent length indicate the distance between the lateralmost endodermal cells and the dorsal site of embryo. Red arrowheads indicate the end of anterior and posterior body axes. D, dorsal. (G,H) Epifluorescence images of anterior endoderm in control, vangl2 mutant Tg(sox17:EGFP) embryos at 4s. Anterior-dorsal view. White lines of equivalent length indicate width of the anterior endodermal sheets. A, anterior; P, posterior. (I) Quantification of endoderm width in each group of embryos shown in (G,H). Number of embryos for each group is indicated. Bars represent the mean±s.e.m. #, p>0.05; student’s t-test. |
|
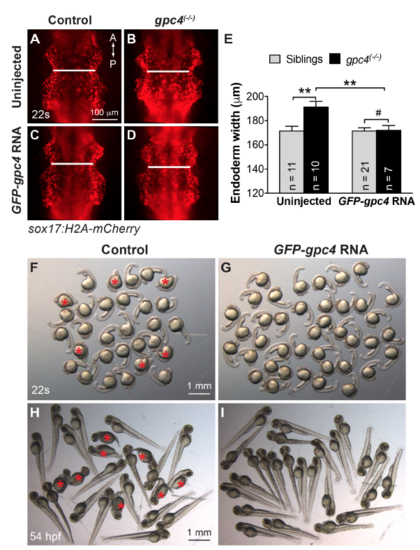
Overexpression of GFP-Gpc4 rescues defects in length of body axis and convergence of anterior endoderm in gpc4 mutant embryos. (A-D) Representative images of indicated Tg(sox17:H2A-mCherry) embryos injected with or without GPF-gpc4 RNA at 22s. Anterior-dorsal view; white lines of equivalent length indicate width of the anterior endodermal sheets. A, anterior; P, posterior. (E) Quantification of the width of the anterior endodermal sheet in each group of embryos shown in (A-D). Data represent mean±s.e.m. The number of embryos is indicated. #, P>0.05; **, P<0.01, student’s t-test.(F-I) Bright-field images of groups of embryos derived from crosses of gpc4(+/-) injected with or without GPF-gpc4 RNA at 22s and 54 hpf. Red asterisks indicate gpc4 homozygous embryos with a short anterior-posterior body axis. |
|
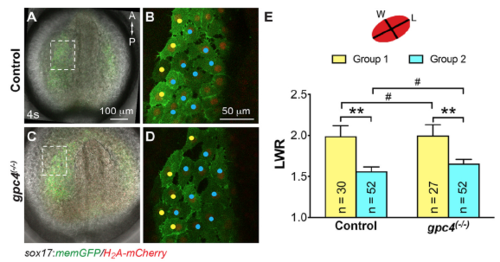
Morphology of anterior endodermal cells is not affected in gpc4 mutants. (A,C) Overlay of bright-field and epifluorescence images of Tg(sox17:memGFP/H2A-mcherry) embryos at 4s. Dashed boxes are regions in which cells were imaged for analysis of shape. (B, D) Confocal images of the endoderm at the region indicated in the dashed boxes in A, C. Endodermal cells at the lateral region and near the dorsal midline are labeled with yellow and cyan dots, respectively. A, anterior; P, posterior. (E) Schematic representation of the method used to measure cell shape (LWR, length-to-width ratio). Quantification of LWR of endodermal cells in seven control and six gpc4 mutant embryos. Bars represent the mean±s.e.m. The number of cells analyzed is indicated. #, p>0.05; **, P<0.01, student’s t-test. |
|
Lam deposition is increased in gpc4 mutant embryos. Transverse cryosections from Tg(sox17:EGFP) control and gpc4 mutant embryos immunostained for Lam (magenta) and nuclei (DAPI, blue). (A-D) Confocal z-stack images of embryos at tailbud (TB) (AB ) and 4-somite (4s) (C-D) stages. Lam assembly between the ectoderm and mesoderm (yellow arrowheads) and around the endodermal layer (white arrowheads). (E) Relative Lam intensity in nonendodermal (Non-end) tissue and around the endodermal layer (End) in control and gpc4 mutant embryos at TB and 4s. The number of embryo analyzed is shown in the graph. Bars represent the mean±s.e.m. *, P<0.05, student’s t-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Effects of suppressing Fn or/and Lam expression on endoderm C&E. (A) Expression of lam1 (a1, b1a, b1b, c1) and fn (1a and 1b) relative to that of the housekeeping gene eukaryotic translation elongation factor 1 alpha 1a (eef1a) in WT embryos at TB and 4s, as determined by qRT-PCR. (B-B) Confocal z-stack images of transverse cryosections immunostained for Lam (magenta) and nuclei (DAPI, blue) from the indicated embryos. (C) Western blot showing expression levels of Fn and Hspa9 (internal control) in embryos indicated. (D-I) Epifluorescence still images of the anterior endodermal sheet in embryos indicated. Anterior-dorsal view. A, anterior; P, posterior. White lines of equivalent length indicate width of anterior endodermal sheet of the embryos at the same stage. (J,K) Average width of anterior endoderm. (J) Embryos injected with the indicated MO (10 ng), shown in (D-F). (K) Embryos treated as indicated and shown in (G-I). Number of embryos analyzed is indicated for each group. #, p>0.05, **, p<0.01, ****, p<0.0001, student’s t-test. PHENOTYPE:
|
|
Mmp14a/b are required for C&E movements of the anterior endodermal cells. (A-B) Confocal z-stack images of transverse cryosections from Tg(sox17:EGFP) control embryos and embryos injected with mmp14a/b ATG MOs (10ng, suppression of translation) immunostained for Fn (magenta) and nuclei (DAPI, blue). Fn assembly at mes/end (white arrowheads) and ect/mes (yellow arrowheads) boundaries. (C-M) Embryos injected with indicated MOs targeting mmp14a/b (ATG MOs target the translation; SP MOs target the splicing). (C-D, F-K) Epifluorescence still images of the anterior region of the endodermal sheet in the indicated embryos. Anterior-dorsal view. A, anterior; P, posterior. White lines of equivalent length indicate the width of anterior endodermal sheets of the embryos at the same stage. (E) Average endodermal width at the anterior region of embryos shown in (C,D). (L) Average width of anterior endoderm in embryos shown in (F-H). (M) Average width of anterior endoderm in embryos shown in (I-K). The number of embryos analyzed in each group is indicated. **, p<0.01; ***, p<0.001; ****, p<0.0001; #, p>0.05, student’s t-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
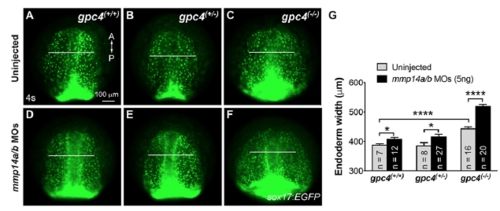
Mmp14a/b and Gpc4 act synergistically in regulating endodermal migration. (A-F) Epifluorescence still images of the anterior endoderm at 4s in embryos derived from crosses of gpc4/Tg(sox17:EGFP) heterozygous zebrafish injected with or without a subdose of mmp14a/b ATG MOs (5 ng). White lines of equivalent length indicate the width of anterior endodermal sheets. A, anterior; P, posterior. (G) Average endoderm width in the anterior region. Numbers of embryos analyzed are indicated for each group. *, p<0.05; ****, p<0.0001; student’s t-test. PHENOTYPE:
|