- Title
-
Sdc2 and Tbx16 regulate Fgf2-dependent epithelial cell morphogenesis in the ciliated organ of asymmetry
- Authors
- Arrington, C.B., Peterson, A.G., and Yost, H.J.
- Source
- Full text @ Development
|
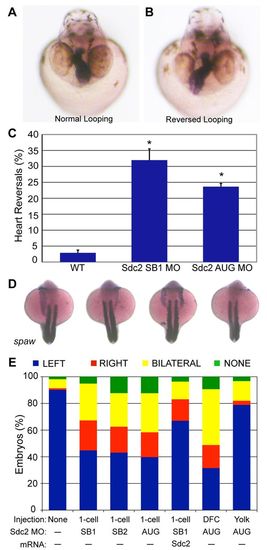
Knockdown of Sdc2 in zebrafish KV causes laterality defects. (A,B) Heart looping is visualized by labeling the heart with an RNA in situ hybridization probe for cardiac myosin light chain 2 (cmlc2) at 40 hpf. (A) Normal heart looping. (B) Reversed heart looping. (C) Both SB and AUG sdc2 morphants had a statistically significant increase in reversed heart looping (SB1 MO: 32%, n=221; AUG MO: 24%, n=369) compared with wild-type (WT) embryos (3%, n=500), *P<0.001. Error bars indicate s.e.m. (D) Examples of morphant embryos at the 18-somite stage displaying left-sided, right-sided, bilateral and absent spaw expression. Somites are labeled with myod1 which is used as a marker for staging embryo development. (E) Expression of Nodal family member spaw is mostly left-sided in wild-type embryos (n=331) but in global sdc2 morphants spaw expression is randomized (SB1 MO, n=215; SB2 MO, n=229; AUG MO, n=190; all P<0.001). Aberrant spaw expression is partially rescued with co-injection of a MO-resistant sdc2 mRNA (n=123; P<0.001 compared with morphants). Targeting MO to the DFCs (DFCsdc2MO embryos, n=147) randomized spaw expression, but targeting MO exclusively to the yolk cell did not (yolksdc2MO embryos, n=181; P<0.001 compared with global and DFC morphants). These results indicate that the role of Sdc2 in LR development is cell-autonomous within the DFCs and that extra-embryonic Sdc2 is not involved in LR development. P-values by Student’s t-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Cell-autonomous regulation of KV morphogenesis by Sdc2. (A-F) Comparison of time-lapse images of uninjected (A-C) and sdc2 morphant (D-F) Tg(sox17:GFP) zebrafish embryos illustrates defective DFC coalescence and KV morphogenesis in sdc2 morphants. KV morphology and size were affected by variation in DFC number and coalescence. (G-J) Anti-ζPKC antibody labels the apical surface of KV cells lining the KV lumen. At the 8-somite stage (SS), KV morphology was normal in wild-type (G) and yolksdc2MO (J) embryos. By contrast, global sdc2 morphants (H) and DFCsdc2MO embryos (I) displayed abnormalities in KV shape as well as incomplete DFC incorporation into the main KV, resulting in aberrant secondary ‘mini vesicles’ (arrowhead in I). (K,L) sox17 expression at 80-90% epiboly reveals tightly clustered DFCs (arrows) in wild-type embryos (K) but loosely adherent DFCs in sdc2 morphants (L). (M,N) Expression of charon is perturbed in sdc2 morphants, indicating KV disorganization. (O,P) pH3 staining in DFCs of wild type [Tg(sox17:GFP)s870] and sdc2 morphants at tailbud to 2-somite stage (see also Table 1). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Cell-autonomous regulation of KV ciliogenesis and asymmetric fluid flow by Sdc2. (A-D) KV cilia labeled with anti-tubulin antibody were shorter in global sdc2 (B) and DFCsdc2MO (C) morphant zebrafish embryos than in wild-type (A) and yolksdc2MO (D) embryos. (E,F) Cilia length and number were reduced in global and DFCsdc2MO morphants (global morphants: seven embryos, 267 cilia; DFCsdc2MO: ten embryos, 177 cilia) compared with wild-type and yolksdc2MO embryos (wild type: six embryos, 373 cilia; yolksdc2MO: six embryos, 287 cilia), *P<0.05 (Student’s t-test). Error bars indicate s.e.m. (G,H) KV fluid flow was altered in sdc2 morphants. Bead tracks are circular in wild-type embryos (G) but disorganized in sdc2 morphants (H). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Sdc2 controls KV morphogenesis through Fgf2 in DFCs/KV. (A,B) In wild-type zebrafish embryos, fgf2 (arrowheads) is expressed (A) in DFCs at 80% epiboly (dorsal view) and (B) in the forming KV at tailbud stage (lateral view). (C) In sdc2 morphants, fgf2 expression is significantly reduced in KV cells (lateral view). (D) fgf2 morphants (2 ng MO) had randomized spaw expression (n=244), similar to that seen in sdc2 morphants (see Fig. 1E). Synergy between Sdc2 and Fgf2 was tested by comparing the effects of subthreshold amounts of each MO individually [4 ng sdc2 SB2 MO (n=431) and 0.5 ng fgf2 MO (n=475)] with co-injection of both MOs. Spaw expression patterns were significantly altered by co-injection of subthreshold doses of sdc2 SB2 and fgf2 MOs [n=486; P<0.001 compared with individual low-dose morphants]. (E,F) fgf2 morphants had smaller KVs (E; labeled with anti-ζPKC antibody) than wild-type embryos, whereas cilia (F, labeled with anti-tubulin antibody) were of normal length. (G,H) Quantification of cilia number and length: cilia number but not cilia length was decreased in fgf2 morphants (wild type: eight embryos, 547 cilia; fgf2 morphants: 11 embryos, 299 cilia), *P<0.001. Error bars indicate s.e.m. P-values by Student’s t-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Sdc2 controls DFC/KV-specific expression of Tbx16, upstream of both Fgf2-dependent KV morphogenesis and Fgf2-independent cilia length regulation. (A-F) KV morphogenesis and cilia length defects in spadetail (spt; tbx16) mutants. (A,C) Normal KV morphology (A) and cilia length (C) in wild type or spt heterozygotes. (B,D) spt mutants had defective KV morphogenesis (B) and cilia length (D), similar to sdc2 morphants. (E,F) Quantification of (E) cilia length and (F) cilia number (spt+/?: eight embryos, 361 cilia; spt-/-: nine embryos, 242 cilia) indicated that both are decreased in spt mutants (*P<0.05, **P<0.001). Error bars indicate s.e.m. (G-L) Sdc2 regulates Tbx16 expression in DFCs/KV. (G,H) Green arrowheads indicate (G) tbx16 RNA and (H) Tbx16 protein expression in DFCs in wild-type embryos. (I,J) Red arrowheads indicate the position of DFCs lacking (I) tbx16 mRNA and (J) Tbx16 protein expression in sdc2 morphants. By contrast, tbx16 RNA and Tbx16 protein expression in adjacent non-DFC domains was normal, revealing a domain-specific regulation of Tbx16 by Sdc2. (K) fgf2 was downregulated in spt mutants (compare with wild type in Fig. 4B). (L) Synergy between Sdc2, Tbx16 and Fgf2 was tested by injection of subeffective amounts of each MO individually (sdc2 SB2 MO, n=431; tbx16 MO, n=396; fgf2 MO, n=475) compared with co-injections of pairs of subthreshold MOs. Co-injection of sdc2 SB2 and tbx16 MOs (n=251) and co-injection of tbx16 and fgf2 MOs (n=111) resulted in significantly affected spaw expression (P<0.001) compared with single injections of subthreshold MOs. P-values by Student’s t-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
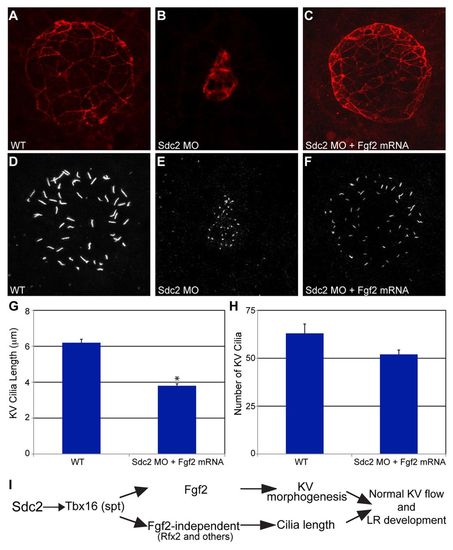
fgf2 mRNA rescues KV morphogenesis and cilia number, but not cilia length, in sdc2 morphants. (A-C) Co-injection of sdc2 AUG MO and fgf2 mRNA rescued KV morphology (labeled with anti-ζPKC antibody). (D-H) Among rescued KV, cilia number in sdc2 morphants was partially rescued by fgf2 mRNA co-injection, but cilia length was not rescued (wild type: seven embryos, 444 cilia; sdc2 morphants + fgf2 mRNA: eight embryos, 416 cilia; compare with sdc2 morphants alone, Fig. 3E,F). *P<0.001 (Student’s t-test). Error bars indicate s.e.m. (I) Proposed genetic pathway by which Sdc2 controls KV morphogenesis and ciliogenesis. DFC-expressed Sdc2 is required for tbx16 expression, which in turn regulates fgf2-dependent KV morphogenesis and fgf2-independent ciliogenesis. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Midline markers lft1, ntl and shh are intact and appear the same as wild-type in sdc2 morphants. (A-F) lft1 (A,B), ntl (C,D) and shh (E,F) in wild type (A,C,E) and sdc2 morphant (B,D,F). |
|
Sdc2 function explored through in situ hybridization and acridine orange staining experiments. (A-C) sdc2 is globally expressed in fgf2 and tbx16 morphant embryos. (D,E) tbx16 is strongly expressed in fgf2 morphants including within DFCs that display disrupted morphogenesis. These data demonstrate that Fgf2 is not required for sdc2 or tbx16 expression. (F,G) Acridine Orange staining was similar in wild type and sdc2 morphants. EXPRESSION / LABELING:
PHENOTYPE:
|