- Title
-
A genetic model of amyotrophic lateral sclerosis in zebrafish displays phenotypic hallmarks of motoneuron disease
- Authors
- Ramesh, T., Lyon, A.N., Pineda, R.H., Wang, C., Janssen, P.M., Canan, B.D., Burghes, A.H., and Beattie, C.E.
- Source
- Full text @ Dis. Model. Mech.
|
Generation of zebrafish transgenics that overexpress wild-type and mutant Sod1. (A) BAC recombineering was utilized to generate the transgenic construct from the zebrafish sod1 gene and surrounding regulatory sequences. The hsp70 promoter was utilized to drive the fluorescent protein DsRed to track transgenesis. (B) Transgene copy number was approximated by quantitative PCR (n=6 per line). (C) Western blots for Sod1 and β-actin with 10 μg of protein from various tissues from G93R os10 and non-transgenic clutch-mate controls (nTg). B, brain; SC, spinal cord; M, muscle; L, liver. (D) Fold change of Sod1 steady-state protein levels in brain homogenates from transgenic lines over nTg control fish. A total of three to six separate samples were quantitated for each line from fish that were 12±2 months of age. Sod1 levels were determined by densitometry and normalized to β-actin. Data are mean ± s.e.m. EXPRESSION / LABELING:
|
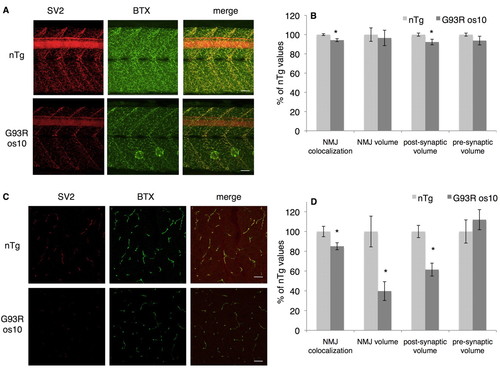
|
Abnormal NMJs are observed in larval and adult G93R os10 fish. (A) Whole-mount antibody labeling with synaptic vesicle 2 (SV2, red) for pre-synaptic vesicles and α-bungarotoxin (BTX, green) for post-synaptic terminals from nTg (top) and G93R os10 (bottom) 11-dpf larvae. (B) Quantitation of NMJ properties in larval (11 dpf) G93R os10 zebrafish in comparison to nTg controls. (C) Adult NMJs were examined in trunk sections by immunolabeling with antibodies to SV2 and α-BTX. (D) Quantitation of NMJ properties in adult (12 month) G93R os10 zebrafish and nTg controls (*P<0.02, n=10 sections from three larvae or adult fish per genotype). Bars, 20 μm. |
|
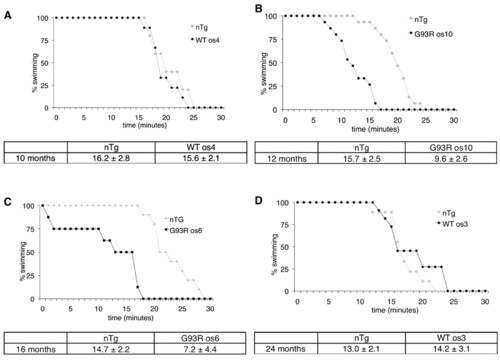
G93R mutant fish exhibit reduced endurance. (A) Ten-month-old adult WT os4 and nTg siblings were subjected to increasing current (4.1 cm/second steps) every 5 minutes until fatigue. Critical swimming speed (Ucrit, cm/s), a measure of the velocity at which a fish can maintain swimming for a set period, was not significantly different between WT os4 and nTg siblings (n=5 nTg and n=9 WT os4). (B) Ucrit values between G93R os10 adults and nTg controls were significantly different at 12 months of age (*P<0.0001, n=15 for each genotype). (C) Affected G93R os6 fish at 16 months of age cannot maintain swimming for as long as nTg controls (*P<0.001, n=10 nTg and n=8 G93R os6). (D) WT os3 fish are unaffected at 24 months of age in comparison to nTg fish (n=9 nTg and n=11 WT os3). Data are mean ± s.d. PHENOTYPE:
|
|
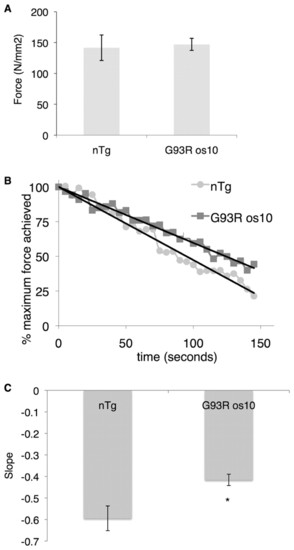
Contractile muscle properties of G93R mutant fish are intact, but the response to repeated stimulation is significantly altered. (A) Muscles of G93R mutant fish are capable of producing similar maximum twitch force as nTg siblings (n=9 per genotype, P=0.816). (B) When subjected to repeated 4 ms stimuli at 0.2 Hz, the muscles of G93R mutant fish fatigue more slowly than those of nTg siblings. The average of the percentage of maximum force achieved (CSA dimension) is plotted against time and linear regression applied to the data (n=8 per genotype). (C) The slope values of linear regression of the percentage of maximal force achieved versus time in response to repeated stimulation were significantly different. G93R mutant fish are able to maintain significantly larger forces for a longer period of time in comparison to nTg sibling controls (n=8 per genotype, P=0.018). PHENOTYPE:
|
|
Motor neuron loss was observed in G93R os10 spinal cord at the disease end-stage. (A) ChAT immunohistochemistry of anterior spinal cord sections from adult G93R os10 fish and nTg siblings demonstrates reduced numbers of positively labeled motor neurons (denoted by arrows) in end-stage fish compared with controls. (B) ChAT-positive motoneurons (≥10 μm diameter) from anterior spinal cord sections (20 μm thickness) of adult end-stage fish were blinded to genotype and counted (*P<0.0001, n=40 sections per fish, three fish per genotype). Data are mean ± s.e.m. Bar, 200 μm. |
|
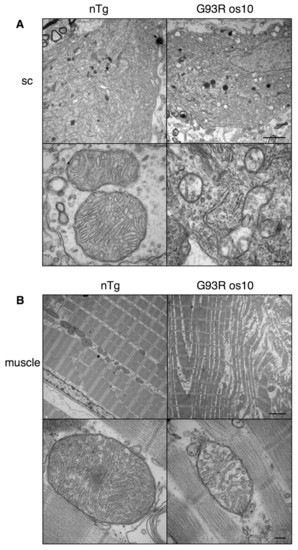
Pathological changes were observed in G93R os10 spinal cord and muscle at the disease end-stage. (A) Electron microscopy of a spinal cord motoneuron from nTg sibling (left) and G93R os10 (right) adult end-stage fish. Numerous vacuolated mitochondria were observed in G93R os10 motoneurons (top) and were readily apparent at higher magnification (bottom). (B) Electron microscopy of trunk muscle from nTg sibling (left) and G93R os10 (right) fish also revealed severe abnormalities (top). The mitochondria in G93R os10 muscle also appeared to be affected, both in number and integrity, and cristae appeared to be reduced in number and lacked the normal organization (bottom). Bars, 2 μm (A,B; top panels); 150 nm (A,B; bottom panels). PHENOTYPE:
|
|
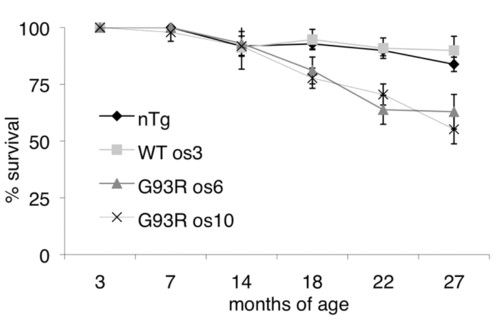
G93R mutant fish have reduced survival. The percentage of surviving fish ± 95% confidence intervals is shown. To track the survival of more than 1000 fish, the numbers of fish were routinely counted. Each time fish were counted, they were placed into an age bracket: 7±2, 14±2, 18±1, 22±2 and 27±2 months of age. At any given age, several independent clutches of fish were counted for each line. See Materials and methods for details on the number of fish counted for each line at each age bracket. PHENOTYPE:
|