- Title
-
A Positive Regulatory Loop between foxi3a and foxi3b Is Essential for Specification and Differentiation of Zebrafish Epidermal Ionocytes
- Authors
- Hsiao, C.D., You, M.S., Guh, Y.J., Ma, M., Jiang, Y.J., and Hwang, P.P.
- Source
- Full text @ PLoS One
|
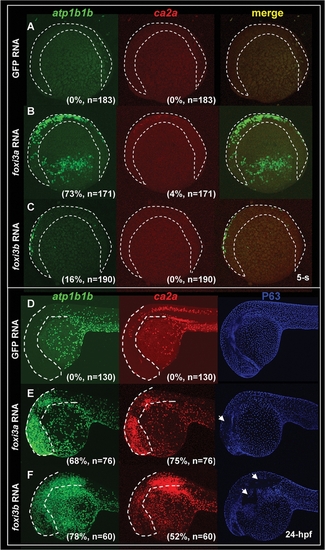
Early Development of Epidermal Ionocytes in Zebrafish Embryos. (A–D) Detection of atp1b1b (green) and ca2a (red) expression in differentiating epidermal ionocytes by fluorescent double in situ hybridization from the 18-somite (18-s) to 72-hour post fertilization (hpf) stage. Note that ca2a is also expressed on spinal cord neurons (sn) and the pronephric duct (pd) in A and B. In A, some epidermal ionocytes which were double-positive for both ca2a and atp1b1b are highlighted with asterisks. (C, D) High-magnification view of ca2a and atp1b1b expressions in the yolk extension region of 72-hpf embryos. Differentiating H+-ATPase rich-cells cells (HRCs) (labeled by asterisks) express a high level of ca2a and a low level of atp1b1b, while differentiating Na+,K+-ATPase-rich cells (NaRCs) are positive for only atp1b1b. (E–M) Dynamic expression of foxi3a (red) and foxi3b (green) in epidermal ionocyte progenitors and differentiating epidermal ionocytes from the 5-s to the 72-hpf stage. Whole-mount in situ hybridization of 72-hpf embryos show that the expression patterns between foxi3a (I) and foxi3b (J) are distinct in the cephalic domain. (K) In the yolk extension region, some cells express a high level of foxi3a (red) and a low level of foxi3b (green), while others are positive only for foxi3b (green). Immunodetection of Na+,K+-ATPase (green) and H+-ATPase (red) in 72-hpf embryos which were in situ-stained with either foxi3a (L) or foxi3b (M). Results showed that foxi3a was only expressed in HRCs (red arrow), while foxi3b was expressed by both HRCs (red arrow) and NaRCs (green arrow). (N) Schematic diagram showing the major events of epidermal ionocyte development in zebrafish embryos at the progenitor stage (the 90% epiboly to 14-s stages), differentiation stage (the 14-s stage to 36 hpf), and maturation stage (from 36 hpf onwards). The developmental stage is indicated in the left lower corner of each panel. EXPRESSION / LABELING:
|
|
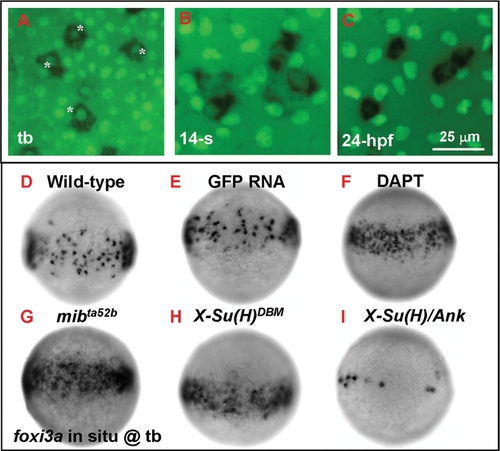
Number of Epidermal Ionocyte Progenitors is Restricted by Delta-Notch-Mediated Lateral Inhibition. (A–C) Dynamic expression of the epidermal ionocyte progenitor marker of foxi3a (black, cytoplasmic staining) and the epidermal stem cell marker of P63 (green, nuclear staining) on the zebrafish epidermis. In the beginning, foxi3a was activated in some P63-positive epidermal stem cells at the tail bud (tb) stage (indicated by asterisks, A). However, as development precedes, P63 expression in epidermal ionocyte progenitors is sharply downregulated (at the 14-somite (14-s) stage, B) ultimately to an undetectable level (24 hour post infection (hpf), C). (D–I) The mosaic distribution of epidermal ionocyte progenitors within the epidermal ionocyte domain is controlled by Delta-Notch-mediated lateral inhibition. In DAPT-treated embryos (F), and mibta52b- (G) or X-Su(H)DBM mRNA-injected embryos (H), the foxi3a expression in the epidermal ionocyte domain has become denser and more homogeneous. While in X-Su(H)/Ank mRNA-injected embryos (I), foxi3a expression in the epidermal ionocyte domain is greatly reduced. The developmental stage is indicated in the left lower corner of each panel. |
|
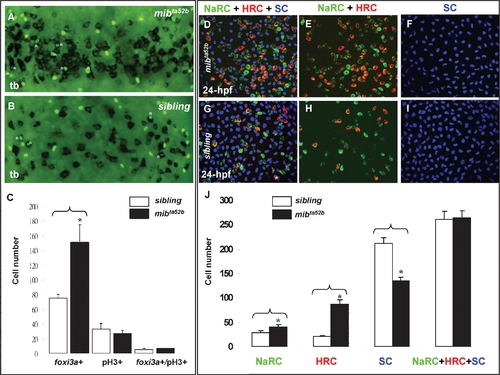
Excess Epidermal Ionocytes Detected in mibta52b Occur at the Expanse of Epidermal Stem Cell Fate. (A–C) Detection of the cell proliferation activity within the epidermal ionocyte domain between mibta52b and siblings at the tail bud (tb) stage. For mibta52b embryos (B) or their siblings (A), epidermal ionocyte progenitors were labeled with foxi3a (black), and mitotic cells were labeled with phosphor-histone 3 (pH3) antibody staining (green). Cells which were double positive for foxi3a and pH3 are highlighted by asterisks. (C) Quantitative comparison of the epidermal ionocyte progenitor number, mitotic divided cell number, and mitotic divided epidermal ionocytes between mibta52b embryos and siblings. (D–I) The excess epidermal ionocytes in mibta52b embryos occur at the expense of the epidermal stem cell fate. For 24-hour post-fertilization (hpf) mibta52b embryos (D–F) and siblings (G–I), differentiating Na+,K+-ATPase-rich cells (NaRCs), H+-ATPase-rich cells (HRCs), and epidermal stem cells were labeled with atp1b1b (green), ca2a, (red), and P63 (blue) staining, respectively. (J) Quantitative comparison of NaRC, HRC, and epidermal stem cell numbers between mibta52b embryos and siblings. The cell number in (C and J) is presented as the mean±S.D. *, p<0.05, compared with siblings, as determined by Student's t-test. |
|
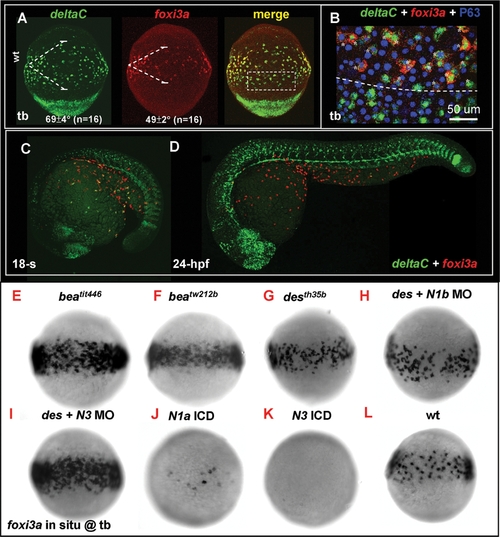
Lateral Speciation on Singling-out Epidermal Ionocyte Progenitors is Mediated by deltaC Ligand and notch1a/notch3 Receptors. (A) Fluorescence double in situ hybridization shows the overlapping expression between deltaC (green, left) and foxi3a (red, middle) on the epidermal ionocyte domain of the ventral ectoderm at the tail bud (tb) stage. The angles of the deltaC and foxi3a expression domain are presented as the mean±S.D. (B) The area demarcated by the dotted line in (A) is viewed at high magnification. Basically, foxi3a+ epidermal ionocyte progenitors (red) also co-express deltaC (green, asterisks). However, some deltaC+ cells outside the epidermal ionocyte domain are negative for foxi3a, suggesting that they are not epidermal ionocytes. (C–D) Fluorescent double in situ hybridization with deltaC (green) and foxi3a (red) probes to show that deltaC is transiently expressed in the epidermal ionocyte lineage. As development proceeds, deltaC is sharply downregulated in the epidermal ionocyte lineage. (E–L) Evaluation of foxi3a expression by genetic mutants or morphants with reduced or enhanced Notch activity at the tb stage. foxi3a expression in the epidermal ionocyte domain was more homogeneous in deltaC mutants of beatit446 (E) and beatw212b (F), in a notch1a mutant of desth35b (G), and in a notch3 MO-injected desth35b mutant (I). The foxi3a expression in the epidermal ionocyte domain was severely reduced in notch1a intracellular domain (ICD) RNA- (J) or notch3 ICD RNA-injected embryos (K). (H) notch1a/des th35b mutants injected with the notch1b MO showed no significant difference with uninjected mutants. N1a, notch1a; N1b, notch1b; N3, notch3; ICD, intra-cellular domain; MO, morpholino. |
|
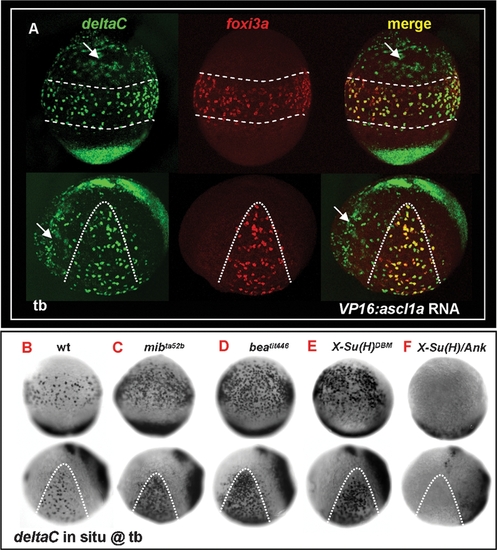
deltaC is Positively Regulated by ascl1a and Receives Negative Feedback by notch. (A) Misexpression of VP16:ascl1a mRNA was sufficient to generate ectopic deltaC expression outside the epidermal ionocyte domain. foxi3a expression, on the contrary, was not affected by VP16:ascl1a mRNA misexpression. (B–F) Evaluation of deltaC expression by genetic mutants or mRNA-injected embryos with enhanced or reduced Notch activity. Compared to the wild-type (B), deltaC expression in the epidermal ionocyte domain was more homogeneous in mibta52b (C), beatit446 (D), and X-Su(H)DBM mRNA-injected embryos (E). On the contrary, deltaC expression in the epidermal ionocyte domain was completely abolished in X-Su(H)/Ank mRNA-injected embryos (F). Embryos in the upper panel of all photos are oriented in ventral view, with the anterior to the top, while in the lower panel, all are oriented in lateral view, with the anterior to the left. Epidermal ionocyte domains are highlighted by dotted lines. hpf, hour post-fertilization; tb, tail bud. |
|
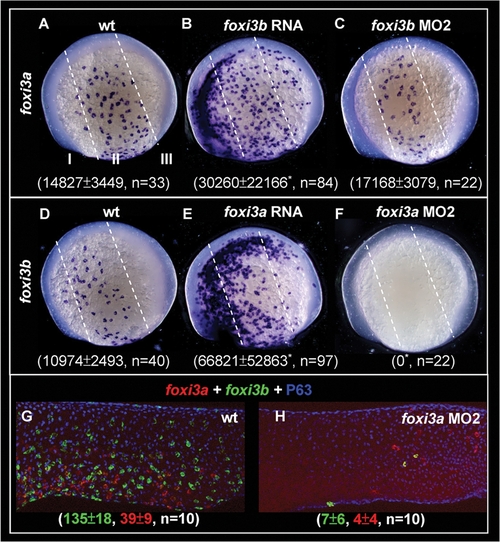
Reciprocal Regulation of foxi3a and foxi3b by a Positive Feedback Regulatory Loop. (A–C) Detection of foxi3a expression in wild-type (wt) embryos, foxi3b mRNA-injected embryos, and foxi3b morphants at the 5-somite (5-s) stage. (D–F) Detection of foxi3b expression in wt embryos, foxi3a mRNA-injected embryos, and foxi3a morphants at the 5-s stage. The foxi3a+ or foxi3b+ area was measured by ImageJ software and is presented as the mean±S.D (µm2). Asterisks indicate p<0.05 when compared with the wild-type embryos, as determined by Student's t-test. To highlight the position of ectopic epidermal ionocytes which were generated by misexpressing either foxi3a or foxi3b, the epidermal ectoderm is subdivided into three domains of I, II and III by dotted lines. (G–H) Comparison of foxi3a (red) and foxi3b (green) expressions between 24-hour post-fertilization (hpf) wt embryos and foxi3a morphants. The number of foxi3a-(red) and foxi3b-(green) expressing ionocytes is indicated in the bottom. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Misexpression of Either foxi3a or foxi3b in a Wild-type Background is Sufficient to Ectopically Generate Precocious Differentiated Ionocytes in the Epidermis. (A–C) Fluorescent double in situ hybridization with atp1b1b (green) and ca2a (red) probes on wild-type embryos, foxi3a mRNA-injected embryos, and foxi3b mRNA-injected embryos aged at the 5-somite (5-s) stage. For wild-type embryos, normal Na+,K+-ATPase rich cell (NaRC) (atp1b1b+) and H+-ATPase rich cell (HRC) (ca2a+ and atp1b1b+) differentiation was not activated until the 14-s and 18-s stages, respectively. However, when foxi3a mRNA was misexpressed in wild-type embryos, it was sufficient to promote the precocious differentiation of both NaRCs and HRCs in the ventral ectoderm and ectopic sites (labeled by a dotted line) at the 5-s stage. For foxi3b mRNA-misexpression, it is only sufficient to promote the precocious differentiation of NaRCs in the ventral ectoderm and ectopic sites (label by dotted line) at the 5-s stage. (D–F) Triple labeling of atp1b1b (green), ca2a (red), and P63 (blue) on wild-type embryos, foxi3a mRNA-injected embryos, and foxi3b mRNA-injected embryos aged at 24 hours post-fertilization (hpf). For wild-type embryos (D), both NaRCs and HRCs seldom appeared on the cephalic ectoderm (highlighted by a dotted line). However, when either foxi3a mRNA (E) or foxi3b mRNA (F) was misexpressed in wild-type embryos, it was sufficient to generate the ectopic NaRCs and HRCs on the cephalic ectoderm. Ectopic epidermal ionocytes occur at the expanse of epidermal stem cell fate, since some regions with strong atp1b1b and ca2a expression completely lacked P63 expression (indicated by arrows). |
|
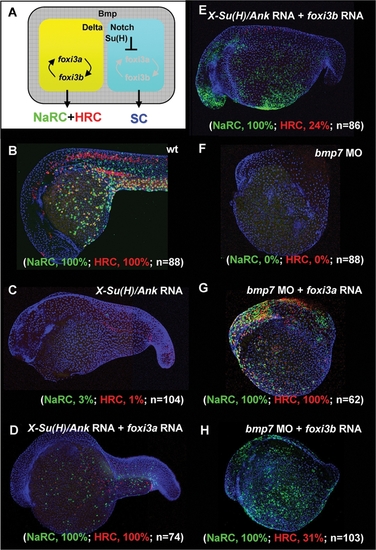
Dissection of the Function of foxi3a and foxi3b in X-Su(H)/Ank mRNA-Injected Embryos or bmp7 Morphants. (A) Schematic diagram showing the role of Bmp and Delta-Notch signaling in specifying the epidermal ectoderm (gray) and singling-out epidermal ionocytes (yellow) from the epidermal stem cell pool (blue). (B) Normal Na+,K+-ATPase rich cell (NaRC) and H+-ATPase rich cell (HRC) differentiation in wild-type embryos aged at 24 hours post-fertilization (hpf). In wild-type embryos, a few IC progenitors were selected from the epidermal stem cell pool within the epidermal ionocyte domain by Delta-Notch-mediated lateral inhibition, and then differentiated into NaRCs (green, detected by atp1b1b in situ) or HRCs (red, detected by ca2a in situ) which are scattered on the epidermal layer. (C) For X-Su(H)/Ank mRNA-injected embryos (500 pg/embryo), the foxi3a and foxi3b expressions were inhibited due to the elevated Notch activity in the epidermal ionocyte domain. In such a condition, all epidermal ionocyte progenitors adopted the epidermal stem cell fate; therefore, subsequent NaRC and HRC differentiation was completely abolished. (D) When foxi3a mRNA (50 pg/embryo) and X-Su(H)/Ank mRNA (500 pg/embryo) were co-injected, high levels of exogenous foxi3a expression could compensate for the elevated Notch activity and restore both NaRC and HRC differentiation. (E) When foxi3b mRNA (50 pg/embryo) and X-Su(H)/Ank mRNA (500 pg/embryo) were co-injected, a high level of exogenous foxi3b expression was also sufficient to compensate for the elevated Notch activity to restore NaRC and partially restore HRC differentiation. (F) In bmp7 morphants (0.1 mM/embryo), although the low level of Bmp signals was still sufficient to promote P63 expression, it was insufficient to drive either foxi3a or foxi3b expression and finally the epidermal ionocytes lost their identity. (G) When foxi3a mRNA (50 pg/embryo) and bmp7 MO (0.1 mM/embryo) were co-injected, a high level of exogenous foxi3a expression could compensate for the low Bmp activity and restore both NaRC and HRC differentiation. (H) When foxi3b mRNA (50 pg/embryo) and bmp7 MO (0.1 mM/embryo) were co-injected, a high level of exogenous foxi3b expression could compensate for the low Bmp activity to restore NaRC and partial HRC differentiation. All embryos were scored at 24 hpf. |
|
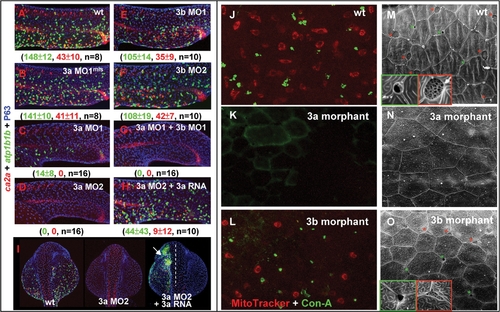
Knock-down foxi3a Expression Severely Reduces Epidermal Ionocyte Progenitor Number and Abolishes the Later Differentiation Program. (A–G) Interfering with foxi3a and foxi3b functions of epidermal ionocyte differentiation by a morpholino (0.5 mM/embryo) injection. Morphants were fixed at 24 hours post fertilization (hpf) and stained with atp1b1b (green), ca2a (red), and P63 (blue) to detect Na+,K+-ATPase rich cells (NaRCs), H+-ATPase rich cells (HRCs), and epidermal stem cells, respectively. (H–I) A rescue experiment to show the specificity of foxi3a morpholinos. The foxi3a mRNA used for the rescue experiment does not contain a binding site for MO2. (J–L) Comparison of the vital dye uptake ability between the wild type (wt), foxi3a morphants, and foxi3b morphants. NaRCs and HRCs in either wild-types or foxi3b morphants can absorb MitoTracker (red) and Con-A (green) through to their apical openings. For foxi3a morphants, no MitoTracker or Con-A staining was detected due to blockage of the entire differentiation program. (M–O) Detection of the apical opening of epidermal ionocytes in wild-types, foxi3a morphants, and foxi3b morphants by scanning electron microscopy. The apical openings of NaRCs and HRCs in wild-types are shaped as deep holes (green box) or a mesh (red box), respectively. The apical openings were totally undetected in foxi3a morphants due to blockage of the entire differentiation program. For foxi3b morphants, the apical openings for both NaRCs and HRCs were reduced. Embryos in (A–I) were scored at 24 hpf, while in (J–O), they were scored at 72 hpf. In I, embryos are orientated in a dorsal-up and anterior-top position. EXPRESSION / LABELING:
PHENOTYPE:
|
|
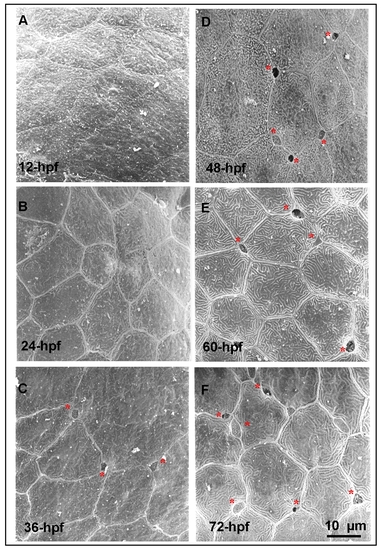
Detection of the apical opening of epidermal ionocytes in zebrafish embryos.(A-F) The epidermal layer covering the yolk ball of wild-type embryos was scanned by a scanning electron microscope at different developmental stages (indicated in the lower left-hand corner). The first apical opening of the epidermal ionocyte appeared at 36 hours post-fertilization (hpf) (asterisks). |
|
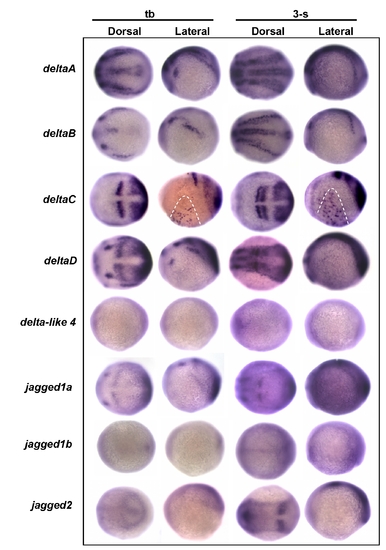
Screening possible delta/jagged ligand expression in the epidermal ionocyte domain. Embryos aged at the tail bud (tb) to 3-somite (3-s) stages were evaluated in situ with either (A) deltaA, (B) deltaB, (C) deltaC, (D) deltaD, (E) delta-like 4, (F) jagged1a, (G) jagged1b, or (H) jagged2 probes. Among the eight delta/jagged genes tested, only deltaC was detected as being expressed on the epidermal ionocyte domain (highlighted by dotted lines) of the ventral ectoderm. |
|
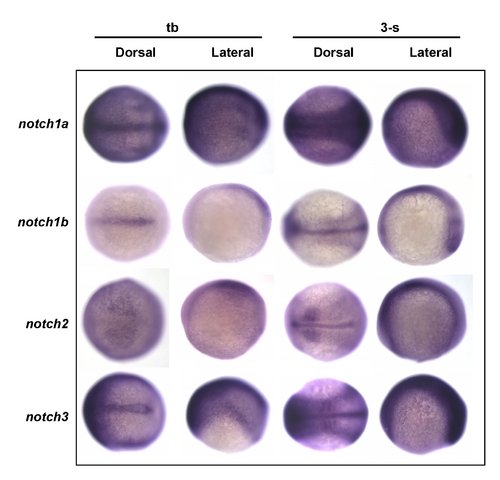
Screening for possible notch receptor expression in the epidermal ionocyte domain. Embryos aged at the tail bud (tb) to the 3-somite (3-s) stages were evaluated in situ with either (A) notch1a, (B) notch1b, (C) notch2, or (D) notch3 probes. Results show that notch1a was strongly and ubiquitously expressed in the epidermal ionocyte domain. Other notch genes, on the contrary, were expressed in the ventral ectoderm at a low level. EXPRESSION / LABELING:
|
|
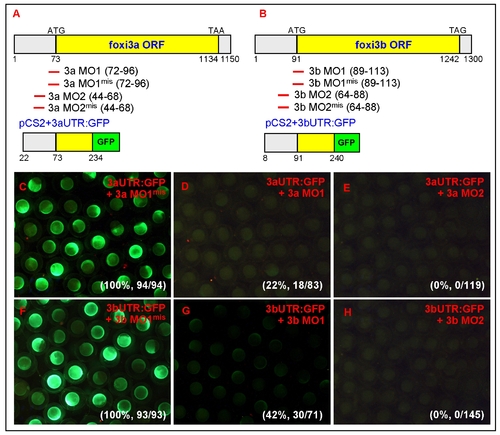
Control experiments to validate the specificity and efficacy of the morpholino. Schematic diagrams show the relative positions of the designed MOs and reporter constructs for either foxi3a (A) or foxi3b (B). The 52UTR and partial exon 1 sequences of foxi3a or foxi3b were PCR-amplified from zebrafish cDNA and in-frame-fused with the green fluorescent protein (GFP) reporter gene. The resulting chimeric vectors of pCS2+3aUTR:GFP or pCS2+3bUTR:GFP contained the complementary sequences for testing the specificity and efficacy of both MO1 and MO2. (C) When five mismatched controls of 3a MO1mis (0.5 mM) were co-injected with 3aUTR:GFP mRNA (250 pg), all embryos (100%, n = 94) showed strong GFP expression. This result suggests that the 3a MO1mis is unable to target endogenous foxi3a mRNA. (D) When the 3a MO1 (0.5 mM) was co-injected with 3aUTR:GFP mRNA (250 pg), GFP expression was completely abolished in 78% of the injected embryos (n = 83). (E) When 3a MO2 (0.5 mM) was co-injected with 3aUTR:GFP mRNA (250 pg), GFP expression was completely abolished in all injected embryos (n = 119). This result suggests that both 3a MO1 and 3a MO2 can target endogenous foxi3a mRNA. However, the efficacy of 3a MO2 was superior to 3a MO1. (F) When five mismatched controls of 3b MO1mis (0.5 mM) were co-injected with 3bUTR:GFP mRNA (250 pg), all embryos (100%, n = 94) showed strong GFP expression. This result suggests that the 3b MO1mis is unable to target endogenous foxi3b mRNA. (G) When the 3b MO1 (0.5 mM) was co-injected with 3bUTR:GFP mRNA (250 pg), it was only sufficient to abolish GFP expression in 58% of injected embryos (n = 71). (E) When the 3b MO2 (0.5 mM) was co-injected with 3bUTR:GFP mRNA (250 pg), it was sufficient to abolish GFP expression in all injected embryos (n = 145). This result suggests that both 3b MO1 and 3b MO2 can target endogenous foxi3b mRNA. However, the efficacy of 3b MO2 was superior to 3b MO1. The number and percentage on the right bottom corner of C-H refer to the ratios of embryos that express GFP. ORF, open reading frame; UTR, untranslated region. |
|
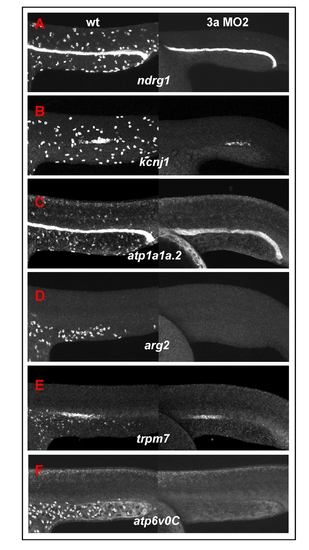
Global down-regulation of epidermal ionocyte markers in foxi3a morphants. (A-F) Comparison of the epidermal ionocyte marker expression between wild-type (left panel) and foxi3a morphants (right panel). The expression of markers is completely abolished in the epidermal ionocyte lineage in foxi3a morphants. Note that the pronephric duct expressions in ndrg1, kcnj1, atp1a1a.2 and trpm7 were largely undisturbed, which shows that the foxi3a morphant phenotype specifically targets epidermal ionocytes. Na+,K+-ATPase-rich cell (NaRC) markers were ndrg1 (A), kcnj1 (B), and atp1a1a.2 (C). H+-ATPase-rich cell (HRC) markers were arg2 (D), trpm7 (E), and atp6voC (F). All embryos were scored at 24 hours post-fertilization (hpf). EXPRESSION / LABELING:
|
|
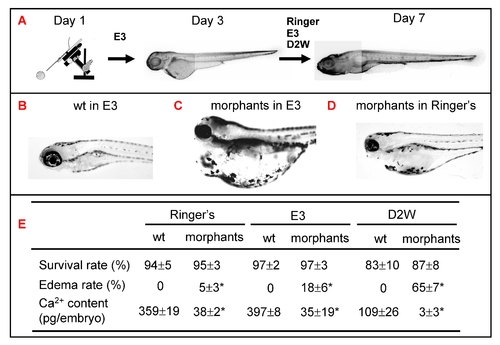
Role of epidermal ionocytes in water and ion homeostasis in zebrafish embryos. (A) Procedure to assay the physiological functions of epidermal ionocytes in zebrafish embryos. After injecting them with foxi3a MO, morphants were initially raised in E3 up to 3 days post-fertilization (dpf) and then challenged with either Ringer's solution, E3, or double-distilled water (D2W). The survival rate, edema rate, and whole-body Ca2+ content between wild-types (wt) and morphants were measured at 7 dpf, and results are summarized in (E). The wild-types had a strong water balance ability and showed no edema phenotype in either E3 (B), Ringer's solution, or D2W (not shown). The morphants displayed a severely edematous phenotype in hypotonic E3 (C) or D2W (not shown), while the abnormality was greatly rescued in isotonic Ringer's solution (D). The values are shown as the mean±SD (n = 10). Asterisks (*) indicate a significant difference from the wild-type (Student's t-test, p<0.05). PHENOTYPE:
|