- Title
-
The development of brain pericytes requires expression of the transcription factor nkx3.1 in intermediate precursors
- Authors
- Ahuja, S., Adjekukor, C., Li, Q., Kocha, K.M., Rosin, N., Labit, E., Sinha, S., Narang, A., Long, Q., Biernaskie, J., Huang, P., Childs, S.J.
- Source
- Full text @ PLoS Biol.
|
All images are captured dorsally and the anterior (A) and posterior (P) axis is marked. ( EXPRESSION / LABELING:
|
|
Nkx3.1 function is required to regulate brain pericyte numbers. Lateral view of control PHENOTYPE:
|
|
Next-generation sequencing analysis of ( EXPRESSION / LABELING:
|
|
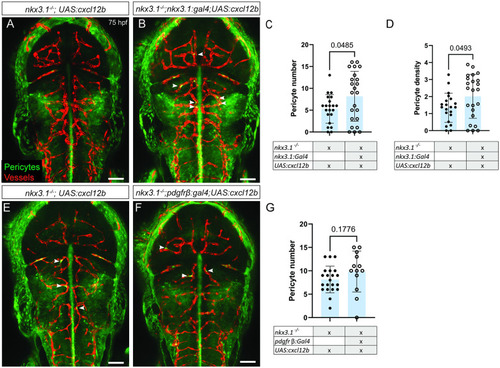
All embryos were imaged dorsally in the head region. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Reexpression of Cxcl12b increases pericyte numbers in All embryos were imaged dorsally in the head region. In comparison to a nkx3.1−/− mutant without a Gal4 driver ( PHENOTYPE:
|
|
nkx3.1 is essential in pericyte precursors. Model of |