- Title
-
Diversity and function of motile ciliated cell types within ependymal lineages of the zebrafish brain
- Authors
- D'Gama, P.P., Qiu, T., Cosacak, M.I., Rayamajhi, D., Konac, A., Hansen, J.N., Ringers, C., Acuña-Hinrichsen, F., Hui, S.P., Olstad, E.W., Chong, Y.L., Lim, C.K.A., Gupta, A., Ng, C.P., Nilges, B.S., Kashikar, N.D., Wachten, D., Liebl, D., Kikuchi, K., Kizil, C., Yaksi, E., Roy, S., Jurisch-Yaksi, N.
- Source
- Full text @ Cell Rep.
|
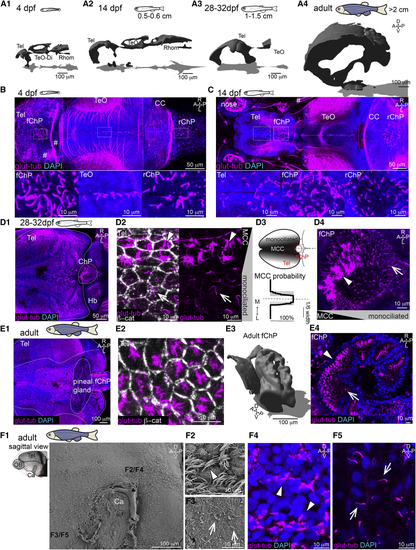
Multiciliation and ventricular/parenchymal expansion correlate during development (A1–A4) Brain ventricles expand during development as shown upon 3D reconstruction of brain ventricles injected with Rhodamine B isothiocyanate (RITC)-dextran. (A1) 4 dpf, n = 4; (A2) 14 dpf, length: 0.5 to 0.6 cm, n = 4; (A3) 28 to 32 dpf, length: 1–1.5 cm, n = 3; and (A4) 2 to 12 months, larger than 2 cm, n = 3. (B) At 4 dpf, single glutamylated tubulin-positive cilia are located on the forebrain choroid plexus (fChP), on the dorsal roof and ventral part of the tectal/diencephalic ventricle, and in the rhombencephalic ChP (rChP) (n = 5). Dashed lines label boundaries between confocal tiles. (C) At 14 dpf, cilia number increases along the dorsal telencephalon, rostral to the fChP, and in the rChP. Cells are monociliated throughout the brain (n = 3). (D1–D4) At 28 to 32 dpf, brushes of glutamylated tubulin-positive cilia appear on the dorsal telencephalon anterior to the ChP and in the ChP (n = 3; D1). (D2) The presence of monociliated (arrow) and MCCs (arrowhead) is shown upon co-staining with the membrane marker β-catenin. (D3) MCCs are located medially to monociliated cells in the tela choroida (TC; quantified in bottom panel; n = 9). (D4) The fChP comprises mono- and MCCs, arranged in an anterior-posterior manner (n = 3). (E1–E4) In the adult brain, MCCs are enriched in the medial part of the TC above the telencephalon (E1) and in the ChP (E4). (E2) Cilia do not cover the entire apical surface of MCCs as shown upon co-staining with β-catenin (n = 4). (E3) Adult fChP consists of multiple interconnected cavities, as shown upon 3D reconstruction of ventricles injected with RITC-dextran (n = 4), and contains mono- and MCCs (E4) (n = 3). (F1–F5) Mono- and MCCs are present on the adult telencephalic/diencephalic midline in the region surrounding the anterior commissure (Ca) highlighted in red. Scanning electron microscopy (n = 3) and immunostaining with glutamylated tubulin (n = 3) show the presence of MCCs (F2 and F4) and monociliated cells (F3 and F5). Location of (F2)–(F5) is indicated in (F1). A, anterior; P, posterior; D, dorsal; V, ventral; M, medial; L, lateral; Tel, telencephalon; TeO, optic tectum; Rhomb, rhombencephalon; CC, cerebellum; MCCs, multiciliated cells. Arrowheads show MCCs, and arrows show monociliated cells. See also |
|
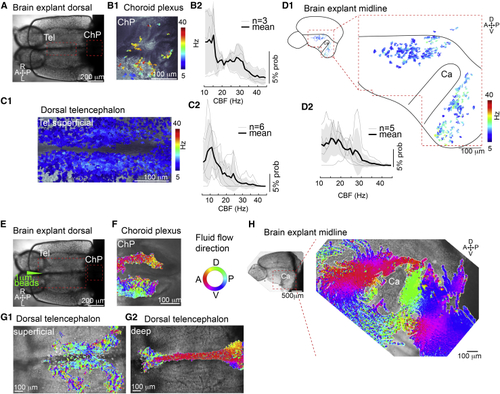
Cilia in the telencephalon, TC, and ChP are motile and generate CSF flow (A) Dorsal view of an adult brain explant. (B–D) Cilia in the forebrain ChP (B), TC (C), and telencephalic midline (D) are motile as shown by analysis of high-speed video microscopy using a pixel-based Fourier analysis. (B1–D1) Map of ciliary beating with ciliary beat frequency (CBF) color-coded in the ChP (B1), TC (C1), and hemisphere explant anterior and posterior to the Ca (D1). (B2–D2) Probability histogram showing the frequency of the pixel-based analysis and the average ± SEM for ChP (B2), TC (C2), and telencephalic hemisphere (D2). n = number of brain explants. (E) Dorsal view of an adult brain explant injected with 1 μm fluorescent beads. (F–H) Directional fluid flow in the ChP (F; n = 4), dorsal telencephalon (G; n = 5), and telencephalic midline (H; n = 5) is color-coded. Tel, telencephalon; A, anterior; P, posterior; D, dorsal; V, ventral; R, right; L, left. See also |
|
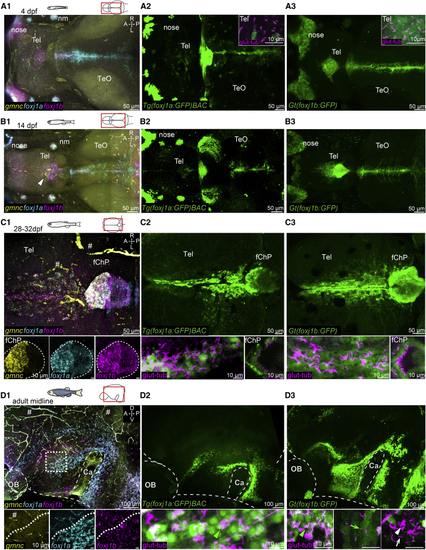
Ciliated cells in the TC, telencephalon, and ChP express (A–D) Expression of (A1–A3) At 4 dpf, (B1–B3) At 14 dpf, there is an expansion of (C1–C3) At 1 month, (D1–D3) On the midline of the adult telencephalon/diencephalon, Number of datasets: A1 = 4, B1 = 4, C1 = 4, D1 = 3, A2 = 4, B2 = 3, C3 = 4, D3 = 4, A3 = 4, B3 = 3, C3 = 4, and D3 = 3. A, anterior; P, posterior; D, dorsal; V, ventral; M, medial; L, lateral; Tel, telencephalon; TeO, optic tectum; OB, olfactory bulb; nm, neuromast. Note that there is unspecific HCR signal associated with blood cells and vasculature (indicated by the hashtag symbol). See also EXPRESSION / LABELING:
|
|
(A and B) Absence of MCCs in 1-month-old (A1 and A2; n = 3 ctrl; 3 mutants) and adult brains of (C1–C3) (D) HCR shows that Asterisk indicates axons. A, anterior; P, posterior; R, right; L, left; Tel, telencephalon. See also EXPRESSION / LABELING:
PHENOTYPE:
|
|
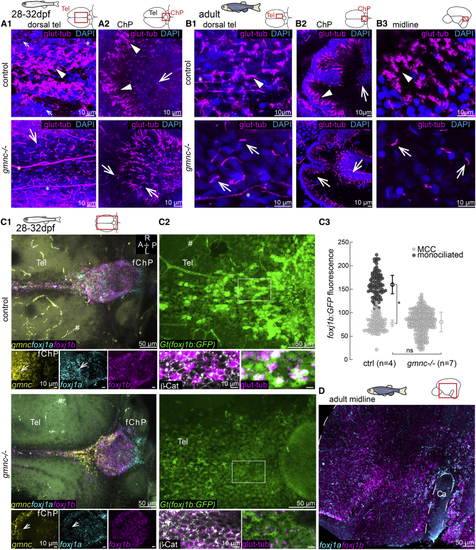
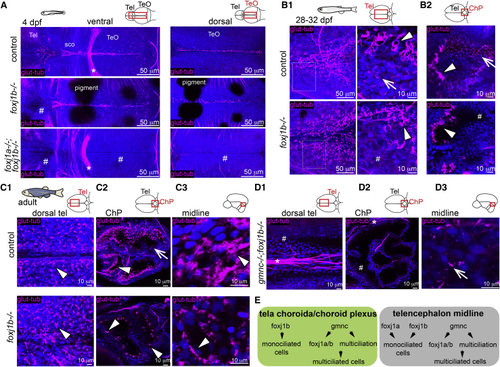
(A) Lack of cilia in the dorsal telencephalon in (B1 and B2) MCCs (arrowhead) are not affected in the (C1–C3) MCCs are not affected in the adult medial TC (C1; n = 3), ChP (C2; n = 4), and midline of the telencephalon/diencephalon (C3; n = 3). (C2) Monociliated cells are particularly affected in the ChP. (D1–D3) Loss of cilia in the medial TC (D1) and ChP (D2) of adult (E) Diverse genetic programs instruct the formation of ciliated cells in the TC/ChP and in the telencephalon midline. TeO, optic tectum; sco, sub-commissural organ. Arrows indicate monociliated cells, arrowheads indicate MCCs, and asterisks indicate axon tracts. Cilia loss is indicated by hashtag symbols. See also |
|
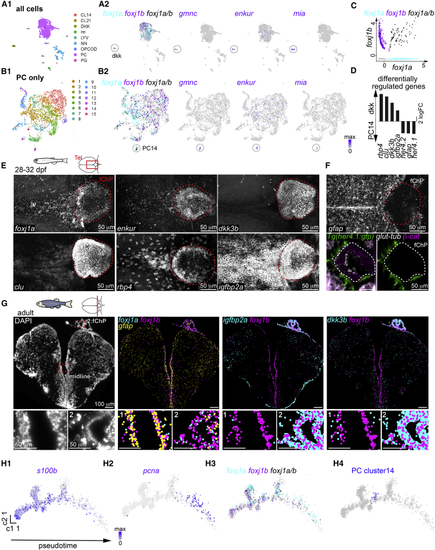
Diversity of motile ciliated cells in the forebrain (A and B) Single-cell RNA sequencing analysis of all cells (A1) or all progenitor-like cells (PCs; B1) revealed the presence of two potential ependymal clusters, (C) Scatterplot representing the expression levels of (D) Selection of differentially regulated genes between the (E) The (F) The PC14 markers (G) Molecular cartography on an adult telencephalic cryosection (DAPI; left) showed that (H1–H4) Pseudotime analysis of the PCs plotted using Monocle algorithm showed a progression from quiescent ( See also EXPRESSION / LABELING:
|
|
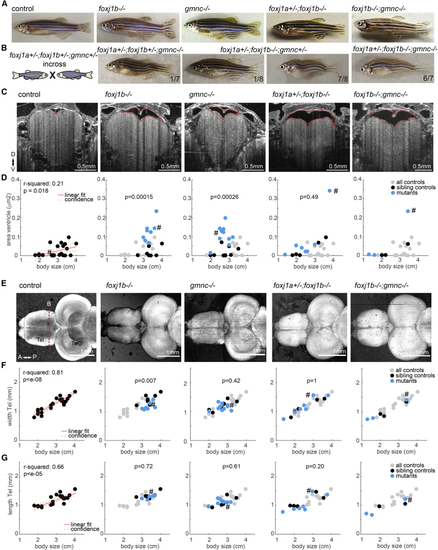
(A) Pictures of adult zebrafish showing absence of scoliosis. (B) Occurrence of scoliosis in some of the progeny of triple (C) OCT images of anaesthetized adult zebrafish revealed enlarged telencephalic ventricles (highlighted with dashed red line) in animals with cilia defects. Transverse section taken as indicated by red dashed line in (D). (D) Quantification of ventricular size in all animals as a function of body length. (E) SD projection of OCT images of adult brain explants showing absence of overt brain malformations in cilia mutants. (F and G) Quantification of the telencephalic width (F) and length (G) as a function of body length. (D, F, and G) R-squared indicate the linear relationship between body length and the measurements as shown in red. Left: all controls pooled. Others: all controls in gray, sibling controls in black, and mutants in blue. p value based on rank sum between sibling controls indicated in black and blue. Examples shown are highlighted in the scatterplot with a hashtag symbol. D, dorsal; V, ventral; A, anterior; P, posterior; Tel, telencephalon; TeO, optic tectum. See also PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

Unillustrated author statements |