- Title
-
The Hippo pathway effector Taz is required for cell morphogenesis and fertilization in zebrafish
- Authors
- Dingare, C., Niedzwetzki, A., Klemmt, P.A., Godbersen, S., Fuentes, R., Mullins, M.C., Lecaudey, V.
- Source
- Full text @ Development
|
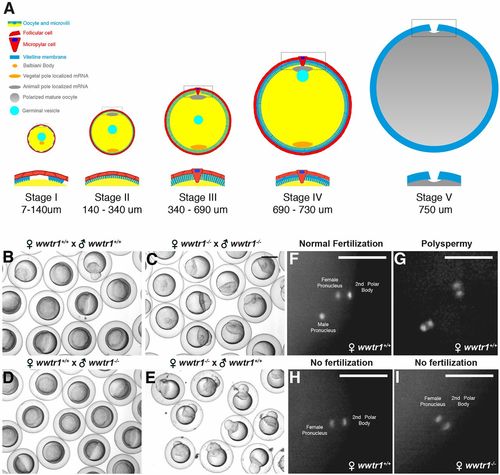
wwtr1 mutant females are infertile. (A) Scheme representing the five different stages of zebrafish oogenesis. (B-E) Eggs or shield stage embryos from a wwtr1+/+ incross (B, N=3), a wwtr1−/− incross (C, N=3), an outcross of wwtr1−/− males to WT females (D, N=3) or an outcross of wwtr1−/− females to WT males (E, N=3) (Table S1). (F-I) Eggs from wwtr1+/+ (F-H, N=2, n=40) or wwtr1−/− females (I, N=2, n=40) fertilized in vitro with WT sperm and stained with DAPI. N, number of experiments; n, number of samples. Scale bars: 50 μm in F-I. PHENOTYPE:
|
|
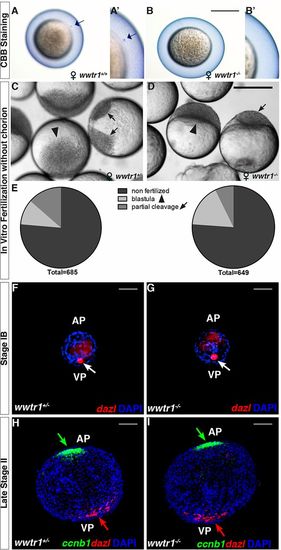
Eggs from wwtr1−/− females do not form a micropyle. (A-B′) The micropyle is stained by Coomassie Brilliant Blue in activated eggs from wwtr1+/+ females (A,A′, n=10) but not from wwtr1−/− females (B,B′, n=8). Arrows indicate the micropyle. (C-E) Blastula (arrowhead) or partially cleaved (black arrows) eggs from wwtr1+/− (C) or wwtr1−/− (D) females fertilized in vitro after enzymatic removal of the chorion and corresponding quantification (E). (F-I) WISH with the Balbiani body marker dazl (F-I, red arrow) and the animal pole marker ccnb1 (H,I, green arrow) in wwtr1+/− (F,H, n=39) and wwtr1−/− (G, I, n=31) oocytes at the indicated stage of oogenesis. FC nuclei are stained with DAPI. AP, animal pole; VP, vegetal pole. Scale bars: 50 μm in F-I; 500 μm in A-D. |
|
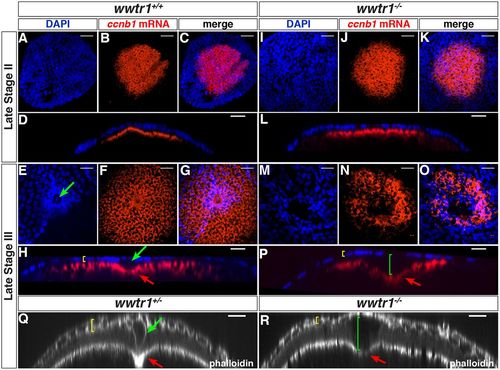
wwtr1 mutant follicles lack the micropylar cell. (A-P) Maximum intensity projection of confocal images of wwtr1+/+ (A-H, n=31) or wwtr1−/− (I-P, n=40) follicles stained by ISH to detect ccnb1 mRNA at the animal pole (red), combined with DAPI nuclear staining (blue) at the indicated oocyte stages. (D,L,H,P) Orthogonal views of corresponding confocal stacks (C,K,G,O). (Q-R) Orthogonal views of confocal stacks of wwtr1+/− (Q, n=9) or wwtr1−/− (R, n=7) follicles stained with Rhodamine Phalloidin. Green arrows indicate an MC; red arrows indicate the indentation of the oolemma; yellow brackets indicate the FCL; green brackets indicate the gap between the FCL and the oocyte surface in wwtr1−/− follicles. The zp0.5:egfp-zorba transgene (not shown) was used to localize the animal pole and orient the follicles. Scale bars: 20 μm. |
|
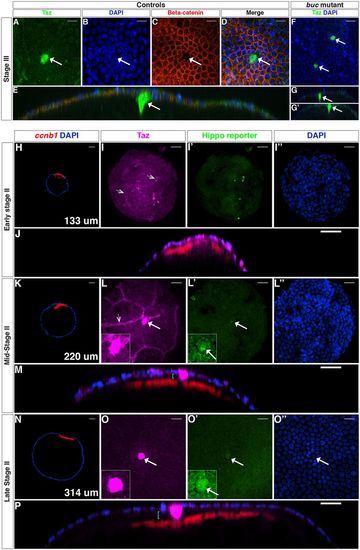
Taz protein is a bona fide marker of the MC. (A-E) Confocal images of stage III WT oocytes stained with anti-Taz (A) and anti-β-catenin (C) antibodies, and DAPI (B, n=4). D shows the three channels merged, and E is an orthogonal view of D. (F-G′) Stage III buc mutant oocytes stained with the anti-Taz antibody and DAPI (n=4). G and G′ are orthogonal views of F. (H-P) Confocal images of wholemount Tg(4xGTIIC:d2egfp)mw50/0 oocytes stained with the anti-Taz antibody (magenta), ccnb1 mRNA (red) and DAPI (blue) at early stage II (H-J), mid-stage II (K-M) and late stage II (N-P) (n=38). Hippo reporter activity is shown in green. Cyan brackets in orthogonal views (M,P) indicate the gap between the oocyte surface and the FC layer (microvilli are not visible); white arrows indicate an MC; dotted arrowheads (I,L) point to non-follicle cells. Insets in L,L′,O,O′ show higher magnification of center of panel. Scale bars: 20 μm in A-D,F,I,J,L,M,O,P; 40 μm in H,K,N. |
|
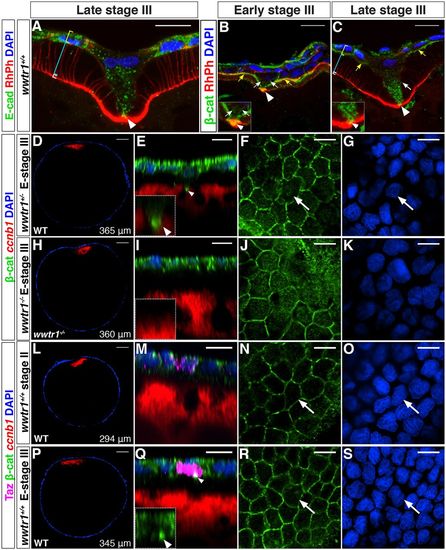
Taz is enriched in the MC precursor before detectable morphological changes. (A-C) Single plane confocal images of cryosectioned ovaries showing E-cadherin (A, n=8) or β-catenin (B,C, n=10) localization in the MC and the neighboring FCs at the contact point (white arrowhead), at the MC membranes (white arrows) and at the interface between the oocyte microvilli and the FCs (yellow arrows). White, cyan and yellow brackets indicate the oocyte cortex, the microvilli/vitelline membrane and the FCL, respectively. Insets (B,C) are higher magnification views of the contact point. Rhodamine Phalloidin (RhPh) and DAPI were used to independently mark membranes and nuclei, respectively. (D-S) Confocal images of wwtr1+/+, wwtr1+/− or wwtr1−/− whole-mount follicles at the indicated stage stained with Taz and/or β-catenin antibodies, ccnb1 mRNA and DAPI as indicated. D,H,L,P are overview images with the follicle size indicated in the lower right corner. White arrowheads in E and Q point to β-catenin enrichment at the contact point; white arrows indicate an MC. E-stage III, early stage III. Scale bars: 10 μm in A-C,E-G,I-K,M-O,Q-S; 50 μm in D,H,L,P. EXPRESSION / LABELING:
|
|
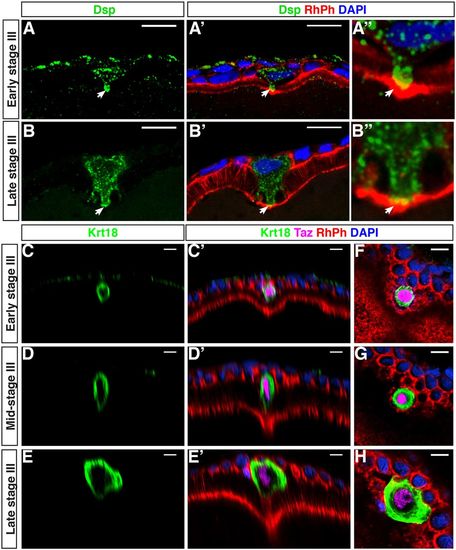
Actomyosin and microtubule cytoskeleton organization the MC. (A-C) Single plane confocal images of cryosectioned ovaries displaying the temporal changes in the localization of Myl12.1-GFP at the animal cortex of the oocyte (N=2, n=34). During stage III, a zone with strongly reduced Myl12.1-GFP forms (white bracket in B,C). (D-E′) Orthogonal views of confocal stacks. Whole-mount follicles stained with an anti-pNMII and Rhodamine Phalloidin (n=27) at the indicated stages of oogenesis. Arrows in E,E’ indicate the localization of pNMII and actin at the lateral membranes of the MC. Rhodamine Phalloidin is used to independently mark the MC membranes (D′-F′). (G-I) Whole-mount follicles stained with an anti-acetylated tubulin antibody to detect stable microtubules in the MC (arrow in H and I) (n=16). (J-O) Whole-mount follicles immunostained with Krt18 (J-K, see Fig. 7 and Results) or acetylated Tubulin (AcTub, N-O) antibodies and counterstained with Rhodamine Phalloidin (RhPh) and DAPI after either cytochalasin D treatment (K, DMSO control in J) (N=2, control n=19, treated n=19) or cold treatment (O, control in N) (N=3, control n=26, treated n=25). (L,M) Snapshots of 3D reconstruction of MCs (see Materials and Methods) from follicles treated with cytochalasin D (M) or with DMSO (L) (see also Movie 2). (P,Q) Quantification of the area of contact between the MC and the oocyte surface (P, P=0.032) or the angle formed at this point (Q, dotted line in N,O; P<0.0001, unpaired two-tailed t-tests). N, number of experiments; n, number of samples. Scale bars: 10 μm in A-O. |
|
Dsp and Krt18 are specifically enriched in the MC. (A-B′′) Confocal images of cryosectioned ovaries stained with an anti-desmoplakin antibody, Rhodamine Phalloidin (RhPh) and DAPI (n=13). White solid arrow points to the contact point between the MC and the oocyte. (C-H) Whole-mount immunofluorescence with an antibody against the intermediate filament protein Krt18 (n=11). C-E′ are orthogonal views of the confocal stacks shown in F-H. Taz antibody, DAPI and Rhodamine Phalloidin were used to counterstain the MC, the nuclei and membranes, respectively. Scale bars: 10 μm. EXPRESSION / LABELING:
|