- Title
-
Stem-loop binding protein is required for retinal cell proliferation, neurogenesis, and intraretinal axon pathfinding in zebrafish
- Authors
- Imai, F., Yoshizawa, A., Matsuzaki, A., Oguri, E., Araragi, M., Nishiwaki, Y., Masai, I.
- Source
- Full text @ Dev. Biol.
|
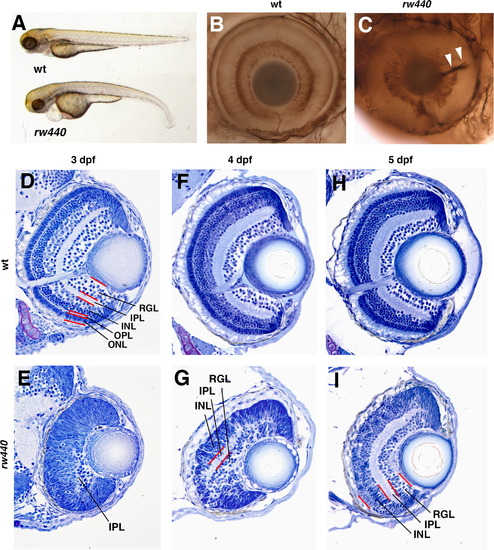
Retinal lamination is delayed in the rw440 mutant. (A) Three dpf wild-type and rw440 mutant embryos. The rw440 mutant embryo shows a small eye, heart edema, and a curled-down body. (B and C) Labeling of 3 dpf wild-type (B) and rw440 mutant retinas (C) with anti-acetylated α-tubulin antibody. Retinal layers are visualized in the wild-type retina. However, retinal lamination is less developed, and retinal axons are misrouted inside the optic cup (arrowheads, C). (D and E) Sections of wild-type (D) and the rw440 mutant (E) retinas at 3 dpf. In wild-type retina, all layers are formed. However, in the rw440 mutant retina, retinal lamination is not observed, except a small IPL. (F and G) Sections of wild-type (F) and rw440 mutant (G) retinas at 4 dpf. In the rw440 mutant retina, the IPL becomes large, and the RGL and INL are distinct. (H and I) Sections of wild-type (H) and the rw440 mutant (I) retinas at 5 dpf. In the rw440 mutant retina, the RGL, IPL, and INL are more evident, but the ONL is absent. RGL, retinal ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer. EXPRESSION / LABELING:
PHENOTYPE:
|
|
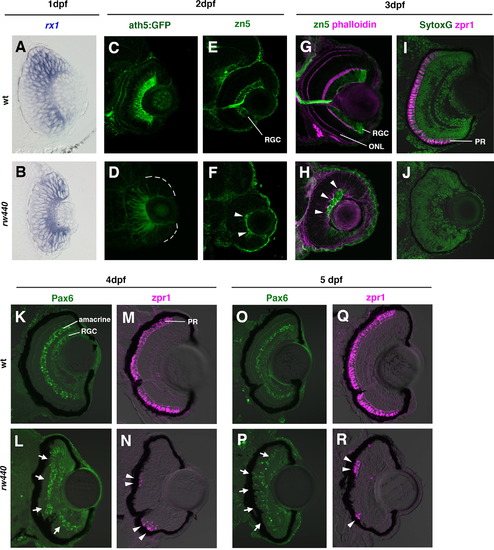
Retinal cell differentiation is delayed in the rw440 mutant. (A and B) Wild-type (A) and rw440 mutant (B) retinas labeled with rx1 RNA probe (blue) at 1 dpf. (C and D) Wild-type (C) and rw440 mutant (D) retinas labeled with ath5:GFP (green) at 2 dpf. In wild-type retina, ath5:GFP is strongly expressed in the RGCs and weakly expressed in other retinal neurons such as amacrine cells and ONL cells. In the rw440 mutant retina, a weak expression of ath5:GFP is observed in the central retina. (E and F) Wild-type (E) and the rw440 mutant (F) retinas labeled with zn5 antibody (green) at 2 dpf. The zn5 antibody labels RGCs in the wild type. In the rw440 mutant, a small number of cells express zn5 (arrowheads, F). (G and H) Wild-type (G) and rw440 mutant (H) retinas labeled with zn5 antibody (green) and phalloidin (magenta) at 3 dpf. Phalloidin labels the IPL and the OPL, making the ONL evident. In the rw440 mutant retina, zn5-labeled RGCs increase (arrowheads), but there is no OPL. (I and J) Wild-type (I) and rw440 mutant (J) retinas labeled with zpr1 antibody (magenta) at 3 dpf. Nuclei were labeled with Sytox Green (green). The zpr1 antibody labels double cone photoreceptors (PR). In the rw440 mutant retina, there is no zpr1 signal. (K and L) Wild-type (K) and the rw440 mutant (L) retinas labeled with anti-Pax6 antibody (green) at 4 dpf. RGCs and amacrine cells express Pax6. In the rw440 mutant retina, presumptive RGCs and some INL cells express Pax6 (arrows, L). (M and N) Wild-type (M) and the rw440 (N) mutant retinas labeled with zpr1 antibody (magenta) at 4 dpf. In the rw440 mutant retina, some ONL cells express zpr1 (arrowheads, N) in clusters. (O and P) Wild-type (O) and the rw440 mutant (P) retinas labeled with anti-Pax6 antibody (green) at 5 dpf. In the rw440 mutant retina, presumptive RGCs and some ONL cells express Pax6 (arrows, P). (Q and R) Wild-type (Q) and the rw440 mutant (R) retinas labeled with zpr1 antibody (magenta) at 5 dpf. In the rw440 mutant retina, clustered ONL cells express zpr1 (arrowheads, R). RGC, retinal ganglion cell; ONL, outer plexiform layer; PR, photoreceptor. PHENOTYPE:
|
|
Cell transplantation experiments. (A) Transplantation of wild-type donor cells (magenta) into wild-type host retinas. Wild-type donor cells incorporated into RGL (arrowheads) express ath5:GFP (green) at 48 hpf. Bottom panels show high magnification. (B) Transplantation of the rw440 mutant donor cells (magenta) into wild-type host retinas. The rw440 mutant cells incorporated into the RGL (arrowheads) did not express ath5:GFP (green) at 48 hpf. Bottom panels show high magnification. (C) Seventy-two hpf wild-type host retinas into which wild-type donor cells (green, upper panels) or the rw440 mutant donor cells (green, lower panels) were incorporated. Retinal sections were labeled with EdU (white) and zpr1 antibody (red). Four sets of right panels are high magnification of the yellow squares shown in the left most panels. Wild-type donor cells into wild-type host retina differentiated into retinal ganglion cells (RGC), amacrine cells (a), bipolar cells (b), horizontal cells (h), and photoreceptors (PR) at 72 hpf. However, the rw440 mutant donor cells did not contribute to RGC and photoreceptors (PR) at 72 hpf, but displayed a progenitor-like, columnar morphology. Some of the rw440 donor mutant cells incorporated EdU (yellow arrowhead, lower right). |
|
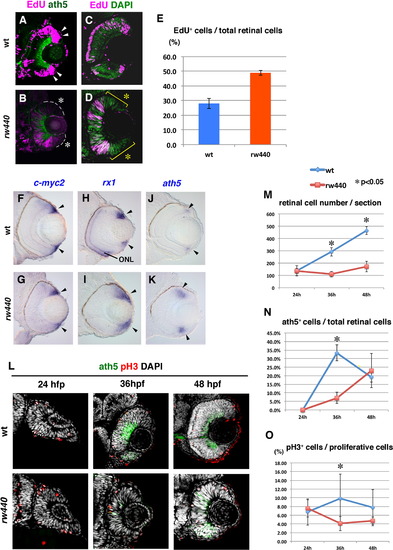
Cell proliferation and subsequent neurogenesis proceed slowly in the rw440 mutant retina. (A and B) Wild-type (A) and the rw440 mutant (B) retinas labeled with ath5:GFP (green) and EdU (magenta) at 60 hpf. In wild-type retina, EdU incorporation is observed in the retinal CMZ (arrowheads, A) and the peripheral ONL of the central retina. In the rw440 mutant, EdU incorporation is not observed in the retinal CMZ (asterisks, B). A weak EdU incorporation was observed in the central retina, and its pattern was complementary to that of ath5:GFP. (C and D) Wild-type (C) and the rw440 mutant (D) retinas labeled with EdU (magenta) and DAPI (green) at 48 hpf. DAPI labels all nuclei. EdU incorporation is observed in the central retina but not in the CMZ (asterisks, D) in the rw440 mutant. (E) Histogram of the percentage of EdU-positive cells relative to total number of retinal cells. Error bars indicate standard deviation. The difference is statistically significant (p<0.05, t-test). (F–K) In situ hybridization of wild-type and rw440 mutant retinas with c-myc2 (F and G), rx1 (H and I), and ath5 (J and K) RNA probes. All three mRNAs were expressed in the retinal CMZ of wild-type and the rw440 mutant retinas (arrowheads). The rx1 is also expressed in the ONL in wild type (H), but not in the rw440 mutant (I). (L) Labeling of wild-type and the rw440 mutant retinas with anth5:GFP (green), anti-pH3 antibody (red), and DAPI (white) at 24, 36, and 48 hpf. (M) Histogram of the number of retinal cells per section in wild-type and the rw440 mutant retinas. (N) Percentage of ath5:GFP-positive cells relative to total number of retinal cells. (O) The percentage of pH3 positive cells relative to proliferative retinal cells. Error bars shown in (M–O) indicate standard deviation. p*<0.05 (t-test). ONL, outer nuclear layer. |
|
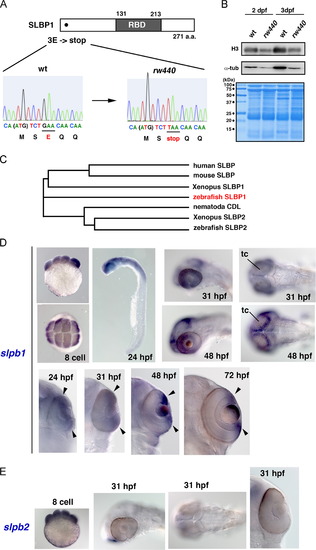
The rw440 mutant gene encodes SLBP. (A) Nonsense mutation occurs in 3-Glutamic acid (E) of the slbp1 gene. RNA-binding domain (RBD) is indicated. (B) Western blot of wild-type and rw440 mutant embryos using anti-histone H3 (top) and anti-α-tubulin antibody (middle). The amount of histone H3 and α-tubulin is decreased in the rw440 mutant at 2 dpf and 3 dpf respectively. The western blot membrane is stained with Coomassie Brilliant Blue (bottom), indicating that the loading amount is the same for each lane. (C) Evolutionary tree of SLBP proteins. (D) Expression of zebrafish slbp1 mRNA. The slbp1 mRNA is expressed in the eight-cell stage, and prominently expressed in the head, including the retina and the tectum at 24 hpf. The slbp1 mRNA expression is gradually restricted in the retinal CMZ (arrowheads), and in the peripheral rim of the optic tectum. (E) Expression of zebrafish slbp2 mRNA. The slbp2 mRNA expression is detected in the eight cell stage, but not after 31 hpf. tc, optic tectum. EXPRESSION / LABELING:
|
|
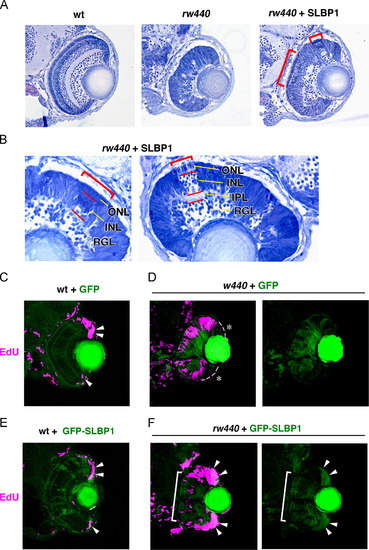
Introduction of SLBP1 rescues cell proliferation and neurogenic defects in the rw440 mutant retina. (A) Sections of wild-type (left), rw440 mutant (middle), and rw440 mutant retinas injected with Tol2[EF1α: SLBP1] (right). Retinal lamination is recovered in columns of the rw440 mutant retinas injected with Tol2[EF1α: SLBP1] (red brackets). (B) High magnification of the rw440 mutant retinas injected with Tol2[EF1α: SLBP1]. Red brackets indicate restored retinal lamination. (C and D) EdU incorporation (magenta) of wild-type (C) and the rw440 mutant (D) retinas injected with Tol2[EF1α: GFP]. EdU incorporation is observed in the CMZ of wild-type retinas (arrowheads, C), but not in the CMZ of rw440 mutant retinas (asterisks, D). The right panel of (D) is the green channel. (E and F) EdU incorporation (magenta) of wild-type (E) and rw440 mutant (F) retinas injected with Tol2[EF1α: GFP-SLBP1]. Expression of GFP-SLBP1 did not have effects on EdU incorporation in wild-type retinal CMZ (arrowheads, E). In the rw440 mutant, expression of GFP-SLBP1 restores EdU incorporation in the retinal CMZ (arrowheads, F), and suppresses EdU incorporation in the central retina (bracket, F). The right panel of (F) is the green channel. RGL, RGC layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer. |
|
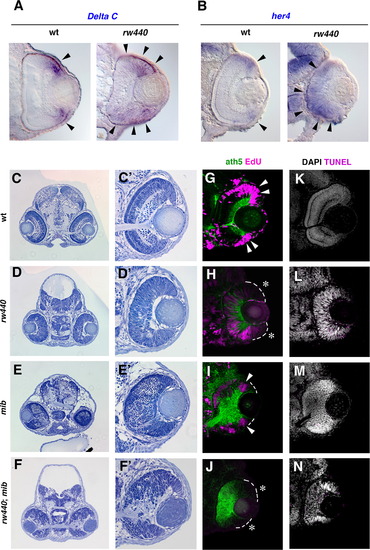
Notch signaling is required for cell proliferation in the central retina of the rw440 mutant. (A) In situ hybridization of wild-type and rw440 mutant retinal sections with Dl-C RNA probes. (B) In situ hybridization of wild-type and rw440 mutant retinal sections with her4 RNA probes. Both Dl-C and her4 mRNA expressions were localized in the retinal CMZ of wild type (arrowheads, left panels), but expanded towards the central retina in the rw440 mutant (arrowheads, right panels). (C–F) Sections of wild-type (C), rw440 mutant (D), mib mutant (E), and rw440; mib double mutant (F) heads at 3 dpf. (C′–F′) High magnification of retinas shown in panels (C–F). (G–J) The ath5:GFP expression (green) and EdU incorporation (magenta) of wild-type (G), rw440 mutant (H), mib mutant (I), and rw440; mib double mutant (J) retinas at 60 hpf. EdU incorporation is observed in retinal CMZ of wild type (arrowheads, G) and mib mutant (arrowheads, I), but not in the retinal CMZ of rw440 mutant (asterisks, H) and rw440; mib double mutant (asterisks, J). EdU incorporation is observed in the central retina of the rw440 mutant but not in the central retina of the rw440; mib double mutant. (K–N) DAPI labeling (white) and TUNEL (magenta) of wild-type (K), rw440 mutant (L), mib mutant (M), and rw440; mib double mutant (N) retinas. Although very faint dot-like TUNEL signals are observed in mib (M) and rw440; mib (N) mutant retinas, there was no massive cell death in both retinas. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The rw440 mutant shows defects in choroid fissure closure and spatial pattern of cxcl12a expression. (A–D) Anti-acetylated α-tubulin antibody labeling of wild-type (A), rw440 mutant (B), mib mutant (C), and rw440; mib double mutant (D) retinas. RGC axons fail to exit from the optic cup, but are misrouted within the optic cup in rw440 mutant retina (arrowheads, B). RGC axons are densely accumulated in the ventro-nasal retina but not misrouted in the mib mutant. RGC axons are more severely misrouted within the optic cup in the rw440; mib double mutant than in the rw440 mutant. (E–H) In situ hybridization of 60 hpf wild-type (E and G), and rw440 mutant (F and H) embryos with the cxcl12a mRNA probe. Ventral (EvF), and lateral (G and H) views are shown. In wild type, cxcl12a mRNA is expressed in the optic stalk, which is located inside the retina and the forebrain region (arrowheads, E and G). However, in the rw440 mutant, cxcl12a mRNA is only detected in the forebrain region (yellow arrowheads, F and H) but not inside the retina. (I–L) In situ hybridization of 60 hpf wild-type (I and K) and rw440 mutant (J and L) embryos with the cxcr4b mRNA probe. Ventral (I and J) and lateral (K and L) views are shown. In both wild type and rw440 mutant, cxcr4b mRNA is expressed in RGCs, although cxcr4b mRNA expression is stronger in the ventro-temporal region in the rw440 mutant (yellow arrowheads, L). (M–Q) In situ hybridization of 60 hpf wild-type (M and O), and rw440 mutant (N, P and Q) embryos with pax2.1 mRNA probe. (N′) High magnification of (N). In wild type, pax2.1 mRNA is expressed in the optic stalk (arrowheads, O). In the rw440 mutant, pax2.1 mRNA is expressed in a reverse V-shape along the ventral surface of the optic cup (yellow arrowheads, N′). (P and Q) Ventral view of deep (P) and superficial (Q) planes of the optic cup. |
Reprinted from Developmental Biology, 394(1), Imai, F., Yoshizawa, A., Matsuzaki, A., Oguri, E., Araragi, M., Nishiwaki, Y., Masai, I., Stem-loop binding protein is required for retinal cell proliferation, neurogenesis, and intraretinal axon pathfinding in zebrafish, 94-109, Copyright (2014) with permission from Elsevier. Full text @ Dev. Biol.