- Title
-
Development of hypothalamic serotoninergic neurons requires Fgf signalling via the ETS-domain transcription factor Etv5b
- Authors
- Bosco, A., Bureau, C., Affaticati, P., Gaspar, P., Bally-Cuif, L., and Lillesaar, C.
- Source
- Full text @ Development
|
5-HT precursors of the embryonic hypothalamus express etv5b. (A-C) Confocal maximum intensity projections of brains from zebrafish embryos processed for 5-HT immunohistochemistry and counterstained with DAPI (confocal optical section). Anterior (a.) and intermediate/posterior (i./p.) 5-HT clusters in the basal forebrain and the raphe population (r.) are indicated. (A,B) Ventral views; (C) lateral view, anterior left. (D-G) Expression of etv5b during embryonic development. etv5b transcripts were detectable in the basal forebrain (bf), at later stages including the hypothalamus (h) and a restricted region of the posterior tuberculum/hypothalamus (arrowheads). Further, transcripts were present in the pineal/epithalamus (e), telencephalon (t), optic stalk (os) and at the midbrain-hindbrain boundary (mhb). Lateral views, anterior left. Inset in F, ventral view. (H) Confocal maximum intensity projection of a Tg(ermp:gv)×Tg(uas:gfp) 30-hpf embryo processed for double gfp/etv5b ISH. Lateral view, anterior left. (I-K) High magnifications of boxed area in H; confocal optical sections. (L-N) Confocal optical section of brain from a Tg(ermp:gv)×Tg(uas:gfp) larvae revealing co-expression of GFP and 5-HT in neurons of the i./p. clusters. Ventral view, anterior left. White arrowheads indicate double-positive cells; open arrowheads indicate cells that are 5-HT-positive only. Scale bars: 50 μm. |
|
etv5b-deficient embryos exhibit fewer 5-HT neurons in the hypothalamus. (A-J) Confocal maximum intensity projections showing controls and etv5b morphants processed for 5-HT immunohistochemistry. The intermediate/posterior (i./p.) clusters of the hypothalamus (A,B,E-G) and the 5-HT population of the anterior raphe (r.) are indicated (C,D,H-J). Ventral views, anterior left. (K-T) nkx2.1a expression in the basal forebrain. Lateral (K,L,O-Q) and ventral (M,N,R-T) views, anterior left. (U,V) The number of 5-HT cells at 72 hpf in controls and etv5b ATG (0.075 mM, U) or splice (0.15 mM, V) morphants expressed as percentage of control. n, total number of embryos analysed for each experiment. *Pd0.05; n.s., not significant. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
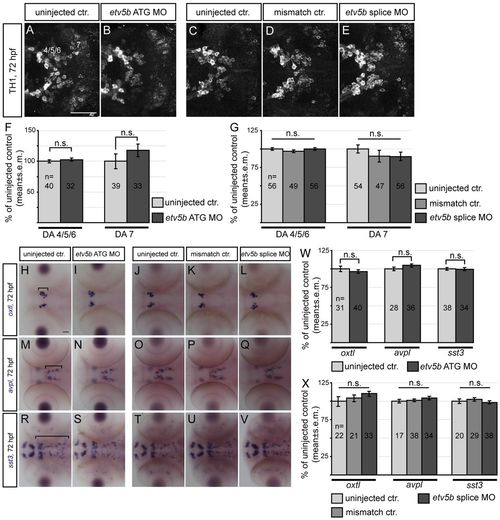
etv5b loss-of-function does not affect the number of cells in other basal forebrain neuron populations. (A-E) Confocal maximum intensity projections showing dopaminergic (DA) neuron clusters (4/5/6 and 7) of the basal forebrain revealed by TH1 immunohistochemistry in controls and etv5b morphants. Ventral views, anterior left. (F,G) The number of TH1-immunoreactive cells in the different DA clusters at 72 hpf in controls and etv5b ATG (0.075 mM, F) or splice (0.15 mM, G) morphants expressed as percentage of control. (H-V) Expression of oxtl, avpl and sst3 in controls and etv5b morphants. Dorsal views, anterior left. Brackets in H, M and R indicate the population included in the analysis. (W,X) The number of cells expressing oxtl, avpl or sst3 at 72 hpf in controls and etv5b ATG (0.075 mM, W) or splice (0.15 mM, X) morphants expressed as percentage of control. n, total number of embryos analysed for each experiment. n.s., not significant. Scale bars: 50 μm. EXPRESSION / LABELING:
|
|
Etv5b is sufficient to induce 5-HT identity within a specific competence field in the hypothalamus. (A-D) Confocal maximum intensity projections showing a control zebrafish embryo and an embryo injected with etv5b RNA and processed for 5-HT immunohistochemistry. The intermediate/posterior (i./p.) clusters of the hypothalamus (A,B) and the 5-HT population of the anterior raphe (r.) are indicated (C,D). Ventral views, anterior left. Scale bar: 50 μm. (E) The number of 5-HT cells in the i./p. and r. clusters at 72 hpf after injection of increasing concentrations of etv5b RNA expressed as percentage of control. n, total number of embryos analysed for each experiment. *Pd0.05; n.s., not significant. |
|
Development of hypothalamic 5-HT neurons depends on Fgf signalling. (A,B) Micrograph showing DMSO control and SU5402-exposed zebrafish embryos processed for etv5b ISH. Following SU5402 treatment, no etv5b transcripts were detected in the hypothalamus (h). Lateral view, anterior left. (C-F) Confocal maximum intensity projections showing control and SU5402-exposed embryos processed for 5-HT immunohistochemistry. The intermediate/posterior (i./p.) clusters of the hypothalamus (C,D) and the 5-HT population of the anterior raphe (r.) (E,F) are indicated. Ventral views, anterior left. (G) The number of 5-HT cells after SU5402 exposure during the indicated time intervals in clusters i./p. and r. at 72 hpf expressed as percentage of control (dashed line at 100%). *Pd0.05; n.s., not significant. (H-O) nkx2.1a expression in DMSO controls and SU5402-exposed embryos. Lateral (H-K) and ventral (L-O) views, anterior left. Scale bars: 50 μm. EXPRESSION / LABELING:
|
|
Etv5b mediates Fgf signalling in the hypothalamus. Rescue experiment in which zebrafish eggs were injected with etv5b RNA and then treated with SU5402 (24-36 hpf). These embryos were compared with the effects of etv5b overexpression alone or SU5402 exposure alone. Embryos exposed to DMSO served as controls. (A-H) Confocal maximum intensity projections showing embryos subjected to the indicated treatments and processed for 5-HT immunohistochemistry. The intermediate/posterior (i./p.) clusters of the hypothalamus (A-D) and the 5-HT population of the anterior raphe (r.) are indicated (E-H). Ventral views, anterior left. Scale bar: 50 μm. (I) The number of 5-HT cells in the i./p. and r. clusters at 72 hpf after the indicated treatments expressed as percentage of control. n, total number of embryos analysed for each experiment. *Pd0.05; n.s., not significant. |
|
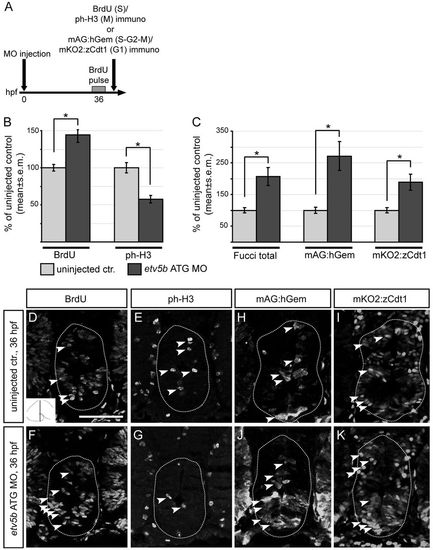
Etv5b regulates cell cycle progression in hypothalamic progenitors. (A) The procedure for analysis of cell cycle kinetics of hypothalamic progenitors. (B,C) The number of BrdU, ph-H3, Fucci total (mAG:hGem and mKO2:zCdt1), mAG:hGem or mKO2:zCdt1 immunoreactive cells at the caudal levels of the hypothalamus at 36 hpf expressed as percentage of control. *Pd0.05. These data suggest that hypothalamic progenitors in etv5b morphants display a longer S phase or are blocked in S phase at 36 hpf. (D-K) Confocal optical sections of coronal cryosections from etv5b morphants or uninjected controls (wild type, D-G; Fucci transgenics, H-K). Wild-type morphants and controls were exposed to a single pulse of BrdU at 36 hpf and processed for BrdU and ph-H3 double immunohistochemistry, whereas Fucci transgenic morphants and controls were processed for mAG:hGem and mKO2:zCdt1 double immunohistochemistry. Sections at the caudal level of the hypothalamus (as indicated in inset in D) are shown. Dotted lines indicate the border of caudal hypothalamus within which cells were counted. Examples of immunoreactive cells are indicated (arrowheads). Scale bar: 50 μm. |
|
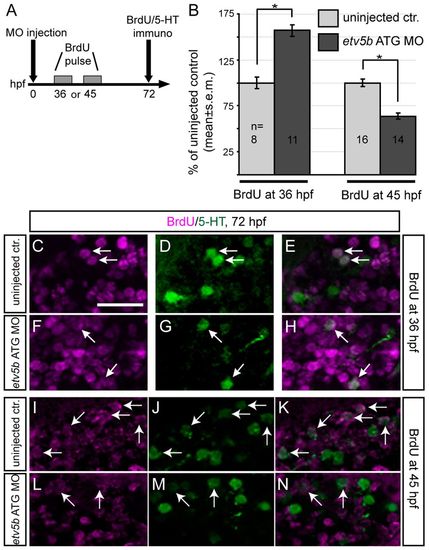
Etv5b is required to keep 5-HT progenitors in a proliferative state and to maintain the pool of 5-HT progenitors. (A) The procedure for analysis of fate at 72 hpf of hypothalamic 5-HT progenitors labelled with BrdU at 36 or 45 hpf. (B) The proportion of 5-HT/BrdU double-positive cells among 5-HT-positive cells in the intermediate/posterior (i./p.) clusters at 72 hpf after a single BrdU pulse at 36 or 45 hpf expressed as percentage of control. These observations suggest that the 5-HT progenitor population, following a transient cell cycle delay, is depleted in etv5b morphants, resulting in an overall decrease in the number of 5-HT neurons at 72 hpf. n, total number of embryos analysed for each experiment. *Pd0.05. (C-N) Confocal optical sections of coronal cryosections from etv5b morphants or uninjected controls. Both were exposed to a single pulse of BrdU at 36 (C-H) or 45 (I-N) hpf and processed for BrdU and 5-HT double immunohistochemistry at 72 hpf. Sections are at the level of the i./p. cluster of 5-HT cells of the hypothalamus. Arrows indicate examples of double-positive cells. Scale bar: 25 μm. EXPRESSION / LABELING:
|
|
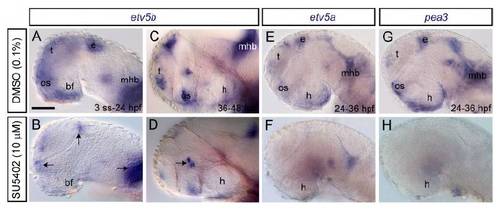
etv5a and pea3 are expressed by the developing hypothalamus but at lower levels/in a more restricted fashion than etv5b. (A-H) Expression of etv5a and pea3 (whole-mounts). At 24 hpf, etv5a transcripts were present in the telencephalon (t), pineal/epithalamus (e), basal forebrain (bf), optic stalk (os), mid-hindbrain boundary (mhb) and rhombencephalon (rh). Progressively, the expression domain in the basal forebrain narrowed, and by 30 and 36 hpf it was present in the ventral/caudal hypothalamus (h). Expression persisted in this region at low levels until 48 hpf. pea3 was expressed in a more restricted manner in the forebrain at 24 hpf, and from 30 hpf onwards its expression in the basal forebrain was limited to a stripe at the distal limits of the hypothalamus. (I-Q) Fluorescent microscopy images of Tg(ermp:gv)3Tg(uas:gfp) transgenic embryos (whole-mount) processed for double gfp/etv5b ISH. Lateral views, anterior left. Scale bars: 50 μm. |
|
etv5b overexpression rescues the 5-HT phenotype seen in etv5b splice morphants. (A) RT-PCR on total RNA extracted from 48 or 72 hpf pooled (n=6), uninjected or mismatch controls as well as embryos injected with increasing concentrations of etv5b splice MO. Injection of the splice MO results in almost complete loss of the wild-type band (wt) and the presence of a morphant band (mo) corresponding to an insertion of the entire intron 10 (79 bp), which in the amino acid sequence results in a premature stop codon. (B-E) Confocal maximum intensity projections showing embryos after RNA rescue of the etv5b splice MO phenotype. Embryos were subjected to the indicated treatments and processed for 5-HT immunohistochemistry (dissected brains). The intermediate/posterior (i./p.) clusters of the hypothalamus are shown. Ventral views, anterior left. Scale bar: 50 μm. (F) The number of 5-HT cells obtained in the rescue experiment in the i./p. and anterior raphe (r.) clusters at 72 hpf after the indicated treatments expressed as percentage of control. etv5b splice MO alone results in a significant decrease in the number of 5-HT-expressing cells as compared with uninjected control siblings (92.3±1.5%, P=6.6×10–4), whereas etv5b RNA injections led to a significant increase (108.3±2.1%, P=2.07×10–3). n, total number of embryos analysed for each experiment. *Pd0.05; n.s., not significant. |
|
Fgf loss-of-function downregulates expression of pea3 family members. (A-H) Embryos were treated with SU5402 during the developmental stages indicated, fixed directly after treatment and analysed for etv5b, etv5a or pea3 expression (whole-mounts). Following SU5402 treatment, no etv5b transcripts were detected in the basal forebrain (bf), including hypothalamus (h), although transcripts were still detectable in some of the domains normally expressing etv5b, including the telencephalon (t), mid-hindbrain boundary (mhb), optic stalk (os) and pineal/epithalamus (e) (B, arrows) and ventral thalamus (D, arrow). SU5402 treatment also abolished etv5a and pea3 expression in the hypothalamus as well as in other domains where they are normally expressed. Lateral views, anterior left. Scale bar: 50 μm. |
|
Hedgehog and Notch signalling are not differentially regulated by Fgf signalling within the hypothalamus during embryonic development. Micrographs showing DMSO controls and SU5402-exposed (24-48 hpf) wild-type embryos processed for ptc1 (A,B) or Notch reporter [Tg(Tp1bglob:eGFP)] embryos processed for gfp ISH (C,D) at 48 hpf (whole-mounts). Insets in A and B show downregulation of ptc1 expression in pectoral fin buds (dorsal view, anterior up). Insets in C and D show gfp signal in hypothalamus after a shorter signal development time. No up- or downregulation of ptc1 or gfp was observed in hypothalamus. Lateral views, anterior left. Scale bar: 50 μm. |
|
Fgf receptors and ligands are expressed in the hypothalamus at a stage when 5-HT progenitors are proliferating. (A-D) fgfr1a, 2, 3 and 4 expression in whole-mount embryos. (E-H) Confocal maximum intensity projections of embryos processed for fluorescent ISH (red) and counterstained with DAPI (blue) covering 30 μm around the midline corresponding to boxed area in A. Transcripts for fgfr1a, 2 and 4, but not fgfr3, were detectable in the caudal hypothalamus (dashed line). (I-N) Expression of fgf3 and fgf8a in whole-mount embryos. At all stages analysed, both transcripts were detectable in the hypothalamus (h). However, fgf3 exhibited a broader expression domain covering the entire caudal/ventral hypothalamus (arrows) and a restricted part of the posterior tuberculum/hypothalamus (arrowheads) (I-K), whereas fgf8a was limited to an area in the most caudal/dorsal hypothalamus (arrows) (L-N). Lateral views, anterior left. Scale bars: 50 μm. |