- Title
-
CaMK-II is a PKD2 target that promotes pronephric kidney development and stabilizes cilia
- Authors
- Rothschild, S.C., Francescatto, L., Drummond, I.A., and Tombes, R.M.
- Source
- Full text @ Development
|
Localization of activated and total CaMK-II in 30 hpf zebrafish embryos. (A-P) Embryos were immunolabeled for total CaMK-II (A,E,I,M) and P-CaMK-II (B,F,J,N) and imaged laterally in the forebrain (A-C), spinal cord cell bodies (E-G), muscle sarcomeres and the pronephric kidney (I-K), and embryonic ear (M-O) were examined (see diagram below). P-CaMK-II colocalizes with acetylated tubulin in some cells and axons in a frontal view of the olfactory placode (D, arrow) and in spinal cord cell bodies (H), but is undetectable in spinal cord axons. P-CaMK-II localizes at the apical surface of pronephric ductal cells (K, arrowhead), at all surfaces of cloacal cells (L) and at the base of the hair cell kinocilium (O, P, arrows). Scale bars: 10 μm EXPRESSION / LABELING:
|
|
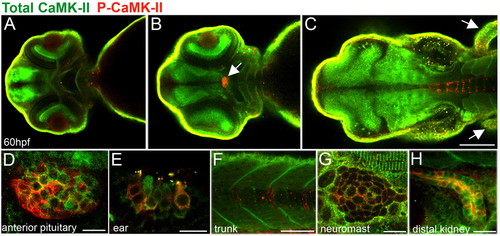
Localization of activated and total CaMK-II in 60 hpf zebrafish embryos. (A-D) Dorsal views of zebrafish brain optical sections (from ventral to dorsal) showing CaMK-II in the forebrain (A), optic nerve and retina and the anterior pituitary (B, arrow, D) and, as expected, in the midbrain, hindbrain (C) and fins (C, arrows). As expected, total CaMK-II is found in the midbrain, hindbrain and fins. (E-F) Lateral views reveal P-CaMK-II in the hair cells of the ear (E), sarcomeres and somite boundaries (F,G), neuromasts (G) and kidney (H). Scale bars: in C, 10 μm for A,B; 10 μm in D,E,G,H; 100 μm in C; 50 μm in F. EXPRESSION / LABELING:
|
|
Activated CaMK-II is enriched in the zebrafish pronephric kidney. (A) Immunoblot of P-CaMK-II and total CaMK-II in whole embryo (6 dpf) and isolated pronephric kidney (3 dpf) extracts demonstrates the preferential activation of CaMK-II in kidney cells. (B-E) P-CaMK-II localization in the anterior, posterior and cloacal cells of the pronephric duct at the lumenal focal plane as shown by counterstaining with acetylated tubulin. P-CaMK-II clusters are marked by arrowheads. (F-H) P-CaMK-II localization at the cell surface of cloacal cells and in cloacal cilia as shown by colocalization with the CAAX-GFP transgenic protein. (I-K) P-CaMK-II localizes at cloacal cilia (revealed by acetylated tubulin). The inset is a z-stack projection of P-CaMK-II in a single cloacal cell. (L-N) Basolateral focal sections of the distal pronephric duct reveals the colocalization of P-CaMK-II with the α1 subunit of the Na+/K+-ATPase at the surface of some but not all transporting epithelial cells. Scale bars: 50 μm in B; 10 μm in D,G,J,M. EXPRESSION / LABELING:
|
|
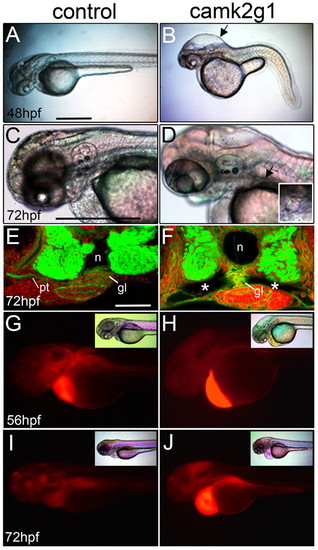
Suppression of γ1 CaMK-II (camk2g1) induces cyst formation. (A,B) Lateral images of 48 hpf morphants injected with the camk2g1 MO (1 ng) but not the control MO (1 ng) show hydrocephaly (arrow) and axis compression. (C,D) Hydrocephaly and cysts are visible at 72 hpf in camk2g1 morphants (inset: dorsal view of anterior cyst (indicated by the arrow). (E,F) Cysts (*) in camk2g1 morphants are revealed in vibratome sections stained with Alexa-Fluor-488-phalloidin and propidium iodide; pt, pronephric tubules; n, notochord; gl, glomerulus. (G-J) Renal filtration was observed in control embryos but not morphant embryos. Rhodamine-dextran was injected into the pericardial region at 56 hpf and then embryos were imaged with DIC optics (inset) or for fluorescence within 20 minutes (56 hpf) and then again at 72 hpf. Scale bars: 500 μm in A,C; 50 μm in E. PHENOTYPE:
|
|
Suppression of γ1 CaMK-II (camk2g1) by injection of the camk2g1 MO (1ng) induces anterior and posterior kidney defects. (A,B) Dorsal view of entire pronephric ducts immunostained for α1 Na+/K+-ATPase; (C,D) regions outlined by white boxes are shown in z-stack projections. (E) Anterior migration is blocked in camk2g1 morphants as inferred from the increase in the distance between the anterior kidney and posterior ear. (F,G) Morphogenic alterations in the ductal region of the pronephros are evident using both DIC optics and GFP-α1 Na+/K+-ATPase fluorescence (insets). (H,I) Pronephric ductal cilia appear normal at all time points as shown by acetylated tubulin immunostaining at 24 hpf. (J,K) DIC images of the cloaca at 48 hpf. Arrows indicate cilia. (L,M) Acetylated tubulin (green) and γ-tubulin (red) show a loss of cloacal cilia but a retention of the basal body (arrowheads) in morphants. (N,O) Cloacal cilia number and length (of remaining cilia) were measured at 24, 48 and 72 hpf. *P<0.005. Scale bars: 100 μm in A,C,F; 5 μm in J,L. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Pronephros specification in camk2g1 morphants. Whole mount in situ hybridization of control and camk2g1 morphants using (A,B) pax2a at 14 somites (dorsal view), (C,D) cdh17, (E,F) gata3, (G,H) ret1 at 24 hpf (C-H, lateral view, anterior to the left); (I-L) wt1a at 24 hpf and 48 hpf (dorsal views). EXPRESSION / LABELING:
PHENOTYPE:
|
|
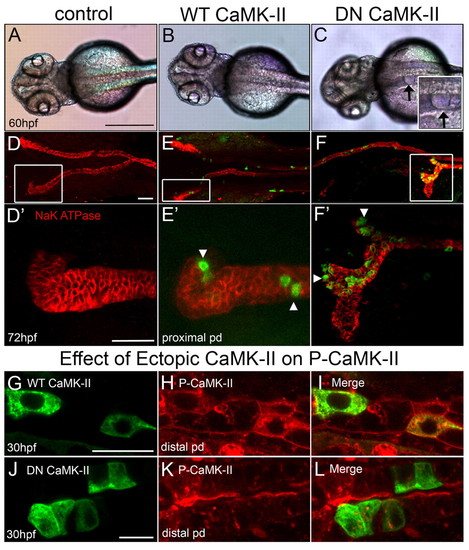
Dominant-negative CaMK-II phenocopies camk2g1 morphants. (A-C) Dorsal views of 60 hpf embryos that were uninjected (control) or injected with kidney-targeted wild-type (WT) and dominant negative (DN) GFP-CaMK-II. Cysts are evident in DN CaMK-II embryos as shown in the inset (arrows). (D-F) Control and WT embryos immunostained for α1 Na+/K+-ATPase undergo proper convolution but DN embryos fail to convolute in proportion to expression (GFP). (D2-F2) Z-stack renderings of the anterior pronephric duct regions outlined by white boxes in D-F. (G-L) Kidney-targeted WT and DN CaMK-II are expressed in pronephric cells, but only DN CaMK-II diminishes P-CaMK-II. Scale bars: 500 μm in A; 20 μm in D,D2 10 μm in G,J. |
|
Active CaMK-II is reduced in pkd2 morphants. (A,C,E) Control and (B,D,F) pkd2 morphants were fixed at 30 hpf and immunolabeled with P-CaMK-II and counterstained for acetylated tubulin. (A,B) P-CaMK-II is reduced in both the distal pronephric duct and cloaca in the morphant. (C) High-magnification image of the distal pronephric duct identifies activated CaMK-II at the apical and basolateral surfaces and in clusters (arrowheads). (D) Activated CaMK-II is lost or greatly diminished in pkd2 morphants. (E,F) P-CaMK-II is reduced, but not absent from cloacal cells in F, but completely lost from cloacal cilia, which are present and normally exhibit activated CaMK-II (inset,*). The insets are z-stack projections of a single cloacal cell stained only with P-CaMK-II. (G,H) Pronephric ducts were dissected from α1 Na+/K+-ATPase-GFP transgenic embryos at 72 hpf. (G) DIC and (H) fluorescence images. (I) CaMK-II enzymatic assay of isolated 3 dpf kidneys shows the reduction, but not elimination of CaMK-II enzymatic autonomy in pkd2 morphants. *P<0.005. Scale bars: 50 μm in A,G; 10 μm in C,E. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Constitutively active (T287D) CaMK-II reverses pronephric developmental defects. (A-F) Lateral views of 72 hpf entire embryos (A-C) and the cloaca (D-F; arrows indicate the end of the cloaca) after injection of pkd2 MO (4 ng) with or without T287D CaMK-II cDNA (30 pg). (G-I) Immunostaining α1 Na+/K+-ATPase identifies the pronephric ducts. (G2-I2) Z-stack renderings of the regions outlined by white boxes in G-I. (J-L) T287D CaMK-II localizes to apical surfaces of pronephric epithelial cells. (M) Quantification of the reversal of the pkd2 morphant phenotype (posterior: occlusion and anterior: convolution) by T287D CaMK-II (n=125-268). (N) Recovery of anterior migration of pronephric epithelial cells by ectopic T287D CaMK-II in pkd2 morphants as assessed by a decrease in posterior ear-anterior kidney distance (in μm; n=50-60). (O,P) Cloacal cilia number and length were determined at 24, 48 and 72 hpf. *P<0.005. (Q,R) Cloacal cilia disassemble but basal bodies remain apical as assessed by co-staining with acetylated tubulin (green) and γ-tubulin (red) at 72 hpf. Scale bars: 500 μm in A; 100 μm in D; 20 μm in G,G2 10 μm in J; 5 μm in Q. PHENOTYPE:
|
|
CaMK-II and PKD2 act in the same molecular pathway. (A-F) Embryos injected with camk2g1 MO (0.25 ng), pkd2 MO (1 ng) or camk2g1 and pkd2 MOs (0.25 ng and 1 ng, respectively) were imaged at 72 hpf from lateral perspectives using DIC optics (A-C) or dorsal perspectives using immunofluorescence microscopy to identify α1 Na+/K+-ATPase (D-F). (G,H) Pronephric occlusions and convolution at 72 hpf (G; n=80-125) and pronephric migration as assessed by posterior ear-anterior kidney distance in µm at 72 hpf (H; n=60-82). (I,J) Cloacal cilia number (I) and cilia length (J) were determined at 24, 48 and 72 hpf. *P<0.005. (K-M) 72 hpf morphants immunolabeled with acetylated tubulin (green) and γ–tubulin (red) show a loss of cloacal cilia but retention of the basal body. Scale bars: 500 μm in A; 20 μm in D; 5 μm in K. EXPRESSION / LABELING:
PHENOTYPE:
|
|
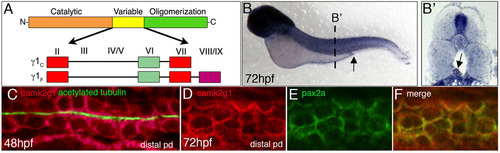
camk2g1 mRNA is expressed in the pronephric kidney. (A) Two alternative splice variants of the γ1 CaMK-II gene (γ1C and γ1F) were identified when cDNA from dissected kidneys or flow sorted kidney cells at 48 and 72 hpf was used as the template for RT-PCR. Alternative exon use in the variable domain is based on previous naming conventions. (B,B2) Whole-mount in situ hybridization was conducted using a γ1C probe at 72 hpf and is shown in whole mounts and in cryosectioned embryos. The cloaca is indicated by an arrow in B and the pronephric duct by an arrow in B2. (C) FISH was performed on 48 hpf embryos with the γ1C probe followed by immunostaining with acetylated tubulin to label pronephric ductal cilia. (D-F) Double fluorescent in situ hybridization demonstrates that γ1C CaMK-II and the pronephric kidney marker pax2a are co-expressed in the distal pronephros. EXPRESSION / LABELING:
|