- Title
-
Critical role of Brg1 member of the SWI/SNF chromatin remodeling complex during neurogenesis and neural crest induction in zebrafish
- Authors
- Eroglu, B., Wang, G., Tu, N., Sun, X., and Mivechi, N.F.
- Source
- Full text @ Dev. Dyn.
|
Brg1 is expressed during early embryonic development. A-D: Whole mount in situ hybridization was performed to detect Brg1 expression in wild-type zebrafish embryos collected at the 1-cell stage, 8-cell stage, shield stage (6 hpf), and 24 hpf. Embryos are dorsal; phenotypes were more than 90%; n = 20 for each embryonic stage. Original magnification 40×. |
|
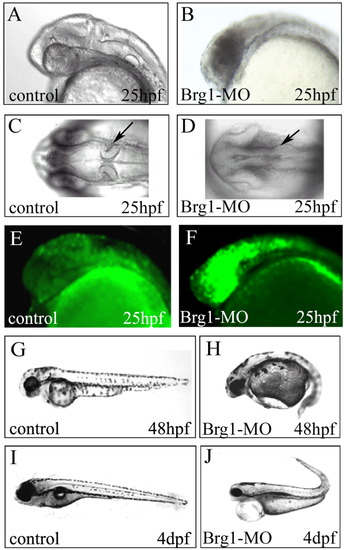
Brg1 expression is critical in neurodevelopment and the proper formation of body axis. A,B: One- to two-cell-stage embryos were injected with control- (A, control) or Brg1-specific MOs (B, Brg1-MO) (10 ng/embryo). Embryos were photographed at 25 hpf. Original magnification 100×. C,D: Dorsal view showing mid/hind-brain boundary under normal conditions (C) or aberrant neural tube development in Brg1-MO-injected embryos at 25 hpf (D). Original magnification 100×. E,F: One- to two-stage embryos were injected with Acridine Orange. Embryos were injected with control-MO (I) or Brg1-MO (J) and photographed at 25 hpf. Morphant phenotypes were more than 90%, n = 30. G-J: Control-MO-injected or Brg1-MO-injected embryos at 48 hpf (E and F) and 4 dpf (G and H). Morphant phenotypes were more than 90%, n = 50. Original magnification 40×. |
|
Reduction in Brg1 expression leads to aberrant expression of genes involved in neurodevelopment. In situ hybridization analyses showing the expression of various markers in control-MO-injected (10 ng/embryo) or Brg1-MO-injected (10 ng/embryo) embryos at indicated time points. The markers were as follows: six3 (A,B), eng2 (C-F), krox-20 (G-J), myoD (K,L), gsc (M,N), ntl (O-R), and hlx1 (S,T). Morphant phenotypes were more than 90%, n = 40. Embryos are dorsal view (A-P) or lateral view (Q-T). For in situ hybridization analyses, a total of 3,000 embryos was injected and 200 embryos were used per probe. No nonspecific hybridization was observed with sense probes (data not shown). Embryos were photographed using a SPOT camera mounted on a dissecting microscope. Morphant phenotypes were more than 90%, n = 30. Original magnification 40×. EXPRESSION / LABELING:
|
|
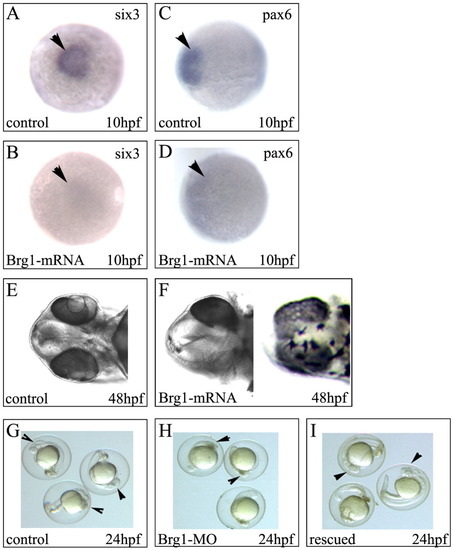
Overexpression of Brg1 mRNA leads to reduced forebrain size and rescues the Brg1-MO knockdown phenotype. A-D: Embryos were injected with 500 pg of full-length Brg1 mRNA at the one- to two-cell stage. Embryos were allowed to recover and, at indicated times, were fixed and used for in situ hybridization using six3- or pax6-specific probes. Arrowheads indicate the areas of affected gene expression. E,F: Control-injected or 500 pg Brg1 mRNA-injected embryos at 48 hpf. Note unequal eye/lens and fused eye/lens in Brg1 mRNA-injected zebrafish embryos (F). The phenotypes of the Brg1 mRNA-injected embryos were approximately 30% each in the two panels in F (n = 200). G-I: Five hundred embryos at the one- to two-cell stage were control-MO-injected (10 ng/embryo) or injected with Brg1-MO (10 ng/embryo), or embryos were injected with Brg1-MO (10 ng/embryo) plus 500 pg of full-length Brg1 mRNA. At 24 hpf, embryos were analyzed, quantitated, and photographed. Arrowheads indicate the head of the embryos. Note small head and body size with signs of apoptosis in H (Brg1-MO treated). With the concentration of the Brg1 mRNA that was used, more than 90% of the embryos could be rescued. Original magnification 30×. |
|
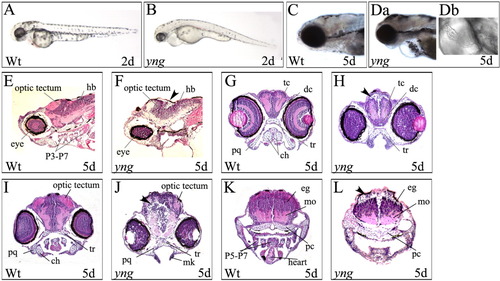
The yng mutant embryos exhibit multiple defects. A,B: yng mutant embryos exhibit lower expression of melanin compared to wild type at 2 dpf. Original magnification 40×. C,Da,Db: yng mutant embryos exhibit cardiovascular defects compared to wild type at 5 dpf. Original magnification 60×. E-L: yng mutant embryos exhibit neurodevelopmental defects compared to wild type at 5 dpf. E and F are sagittal sections. Arrow indicates position of the cerebellum. G-L are coronal sections showing evidence of cell loss in yng mutant embryo (arrowheads). Histological sections were stained with hematoxylin and eosin. hb, hindbrain; P3-P7, pharyngeal arches 3-7; tc, telencephalon; dc, diencephalons; tr, trabeculae; pq, palatoquadrate; ch, ceratohyal; mk, Meckel's cartilage; P5-P7, pharyngeal arches 5-7; pc, parachordal; mo, medulla oblongata; eg, eminentia granularis. Original magnification 200×. |
|
Brg1-MO-injected embryos exhibit reduced expression of genes critical for neural crest induction. In situ hybridization analyses showing the expression of indicated genes in control-MO-injected (10 ng/embryo) or Brg1-MO-injected (5 or 10 ng/embryo), or yng mutant embryos at 12 hpf or the 15-somite (15s) stages. The expression of foxd3, tfap2a, and snail2 were analyzed. Morphant phenotypes were more than 90%, n = 40. Dorsal view of embryos showing expression of foxd3 (A-D,M,N), tfap2a (E-H), and snail2 (I-L). For the in situ hybridization analyses, a total of 2,000 embryos was injected and 100 embryos were used per probe. No nonspecific hybridization was observed with sense probes (data not shown). Original magnification 40×. EXPRESSION / LABELING:
|
|
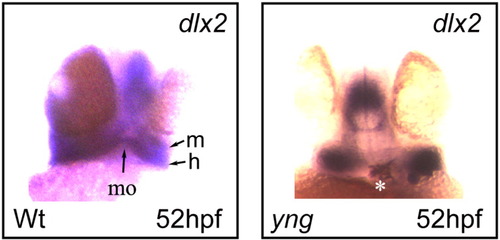
Expression of dlx2 gene, a specifier for neural crest migration. In situ hybridization analyses showing the expression of dlx2 gene in wild type (Wt) and yng mutant embryos at 52 hpf. Note dlx2 expression in the extending mandibular (m) and the hyoid (h) arches positioned in a ventral-anterior direction in the wild type versus ventral in yng mutant. Morphant phenotypes were more than 90%, n = 30. Original magnification 40×. EXPRESSION / LABELING:
|
|
The yng mutant embryos show defects in neural crest cell differentiation. Craniofacial skeletons and schematic drawings of wild type (A,C,E and B,D,F), and yng mutant (G,I,K and H,J,L), embryos following Alcian Blue staining at 5 dpf. The lateral (A and G) and ventral (C and I) views show that all the pharyngeal cartilages form ventrally and the most posterior arches (P6-P7) are reduced or absent in yng mutant embryos. The dorsal processes of the platoquadrate (pq) fail to articulate with the ethmoid plate (ep). The basihyal (bh) along the midline is reduced. The dorsal view of neurocranium (E and K) shows that neural crest-derived ethmoid plate (ep) and mesodermally derived parachordals (pc) are reduced. bh, basihyal; ch, ceratohyal; ep, ethmoid plate; hs, hyosymplectic; mk, Meckel's cartilage; nc, notochord; pc, parachordal; pq, platoquadrate; tr, trabeculae; Wt, wild-type. Note that eyes were removed from the wild type embryo (A) to observe fine details. Original magnification 200×. |
|
The Brg1-MO injected and yng mutant embryos exhibit reduced peripheral neurons and axons. A: One- to two-cell stage embryos were injected with control-MO or Brg1-MO, and at 24 hpf embryos were fixed and immunostained with antibodies to acetylated tubulin (acTUB) (lateral) or HuC/D (dorsal). Original magnification 200×. B: The yng mutant embryos were fixed and immunostained at 5 dpf as above. Lateral views are presented. The acTUB and HuC/D were detected using AlexaFluor 594- and AlexaFluor 488- labeled secondary antibodies, respectively. Image analysis was performed with conventional fluorescent or confocal microscopy. In A, note the reduced primary motor nerves and Rohon Beard sensory neurons in Brg1-MO-injected embryos compared to control (arrowheads). In B, note the reduced DRGs in the trunk region of yng mutant embryos compared to wild type indicated by arrowheads. Original magnification 200×. |
|
Brg1 is recruited to the snail2 promoter in developing zebrafish. A: Wnt signaling pathway is intact in Brg1-MO-injected embryos. The cDNAs were prepared from embryos at indicated times and the expression of Wnt1, Wnt4, Wnt8b, Wnt10b, Frizzled2, β-catenin, LEF1, TCF1, Engrailed2, Snail2, Brg1, and β-Actin was determined using specific primers. Note that the expression analyses of the indicated cDNAs in the wild-type are similar to previous reports (Thisse et al.,[1995]; Dorsky et al.,[1999]; Bellipanni et al.,[2006]) and zfin.org. B: Immunoblot analyses of human Brg1 in developing zebrafish. Plasmids containing human Brg1 cDNA (pcDNA3-Brg1) (De La Serna et al.,[2000]) were injected into one-cell stage embryos. At 22 hpf, lysates were prepared and used to detect Brg1 expression using antibody to human Brg1. Note that we injected plasmids containing human Brg1 because the commercially available Brg1 antibody does not recognize the zebrafish Brg1, although there are high levels of sequence homology between Brg1 cDNA from zebrafish and other organisms. Hela cell lysate was used as a positive control. Lane termed "negative control" represents lysates prepared from zebrafish injected with empty plasmids. β-actin was used as a loading control. C: Human Brg1 efficiently rescues Brg1-MO-injected zebrafish embryos. One-cell stage embryos were left uninjected (top), or injected with Brg1-MO (middle) or Brg1-MO and plasmids containing human Brg1 (50 ng/μl) (bottom). Embryos were photographed at 25 hpf. D: Snail2 promoter regions a and b contain 3 TCF/LEF binding sites (at -1,796 bp and -2,070 bp in a, and at -2,682 bp in b) that were PCR amplified (primers location are indicated by arrows) in ChIP assays. E: Embryos were injected with control plasmids or plasmids containing pcDNA3-human Brg1 at one-cell stage. At 12 and 22 hpf, embryos were collected and prepared for ChIP assays as described in Experimental Procedures. Immunoprecipitated chromatin (or total genomic DNA in the case of input) from each group was PCR amplified for the indicated promoter fragments. Top panels represent where antibody to Brg1 was used to immunoprecipitate chromatin from embryos injected with plasmids containing human Brg1. Middle panels represent where antibody to Brg1 was used to immunoprecipitate chromatin from embryos injected with control empty plasmids. Bottom panels represent PCR products from total genomic DNA input. EXPRESSION / LABELING:
|