- Title
-
Perp is required for tissue-specific cell survival during zebrafish development
- Authors
- Nowak, M., Köster, C., and Hammerschmidt, M.
- Source
- Full text @ Cell Death Differ.
|
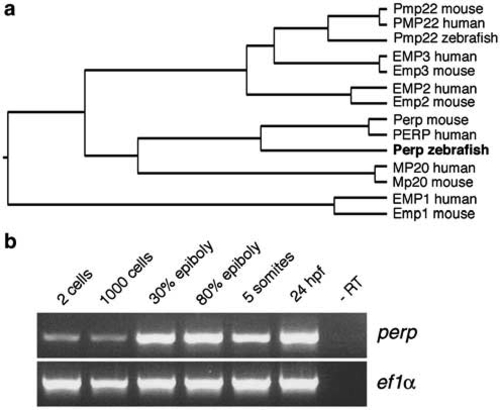
Zebrafish Perp is a member of the PMP22 family and expressed throughout zebrafish development. (a) Phylogenetic tree between different zebrafish, mouse and human members of the PMP22 family, assembled by the Jotun Hein method (DNASTAR Inc.), revealing that the zebrafish protein encoded by cloned cDNA is the Perp ortholog. (b) Semiquantitative RT-PCR analysis of zebrafish perp expression during embryonic development. As control, transcripts of the ef1α housekeeping gene19 were amplified. perp transcripts can be detected during all investigated stages, indicating maternal and zygotic expression of the perp gene |
|
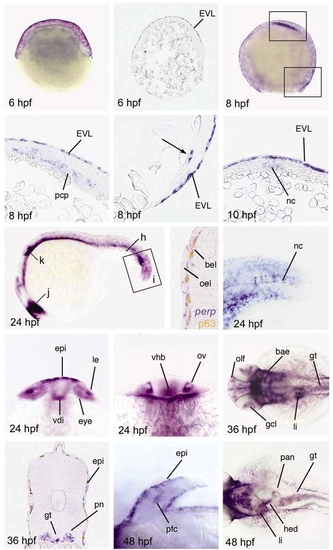
perp is expressed in specific cells of the zebrafish embryo. In situ hybridization with perp antisense riboprobe at different stages of development. (a,b) Shield stage (early gastrula; 6 hpf). (a) Whole-mount embryo, (b) Sagital section. (c-e) 80% epiboly (midgastrula; 8 hpf). (c) Whole mount embryo. (d,e) Longitudinal sections, anterior up, arrow in (e) indicates single deep cells at the dorsal margin. (f) Tailbud stage (end of gastrulation; 10 hpf), transverse section through dorsal midline, dorsal up. perp is expressed in the notochord anlage (nc). (g-k) 25-somite stage (24 hpf). (g) Lateral overview over entire embryo; positions of section (h), magnified region (i), and optical cross-section (j,k) are indicated. (h) Transverse section through the tail of embryo stained for perp mRNA (in blue) and p63 protein (in brown), close-up on the skin. (i) Magnified view on the tail tip. perp shows prominent expression in the notochord. (j) Optical cross-section at level of eyes. (j) Optical cross-section at the level of the otic vesicles. (k,l) 36 hpf. (l) Dorsal view on the head. (m) Transverse section through trunk. (n,o) 48 hpf. (n) Higher magnification of pectoral fin. (o) Dorsal view on trunk. Abbreviations: bae, branchial arch epithelium; bel, basal epidermal layer; EVL, enveloping layer; epi, epidermis, skin; gcl, ganglion cell layer of retina; gt, gut; hed, hepatic duct; le, lense; li, liver; nc, notochord; oel, outer epidermal layer; olf, olfactory epithelium of nasal pits; ov, otic vesicle; pan, pancreas; pcp, prechordal plate; pfc, pectoral fin cartilage; pn, pronephric duct; vdi, ventral diencephalon; vhb, ventral hindbrain |
|
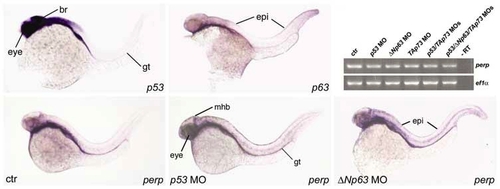
During normal zebrafish development, perp is expressed independently of p53 and the related proteins ΔNp63 and TAp73. (a,b,d-f) show whole-mount in situ hybridizations at 36 hpf; (a) p53 expression, which is most prominent in the brain, eyes, and gut. (b) p63 expression, most prominent in the skin. (c-e) perp expression in uninjected control embryo (c), and in p53 morphant (d), and ΔNp63 morphant embryo (e). perp expression in eyes, midbrain-hindbrain boundary region (mhb), and gut (gt) of p53 morphant, and in the skin (epi) of ΔNp63 morphant are indicated. (f) Comparative semiquantitative RT-PCR analysis of perp transcript levels in p53 morphants (lane 2), ΔNp63 morphants (lane 3), TAp73 morphants (lane 4), p53/TAp73 double-morphants (lane 5) and p53/Tap73/ΔNp63 triple-morphants (lane 6) at 48 hpf, with ef1α cDNA amplification as control. Efficiency of used p53 MO was confirmed by parallel coinjections with mdm2 MO, leading to a rescue of the mdm2 morphant phenotype, as described elsewhere.20 Efficiency of ΔNp63 and TAp73 MOs was confirmed by the skin and craniofacial phenotypes of injected sibling embryos at later stages of development23, 26 |
|
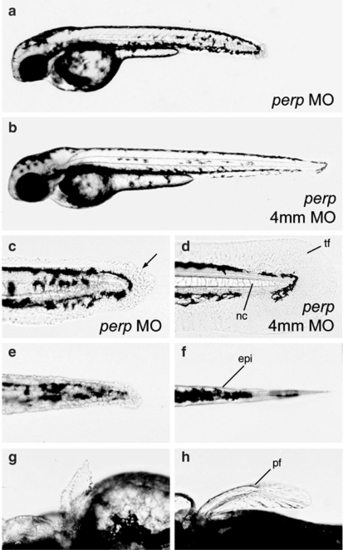
Loss of Perp causes tissue degeneration. All images show live embryos at 48 hpf, either injected with a perp-specific antisense MO (perp MO) (a,c,e,g), or the four mismatch control MO (4 mm) (b,d,f,h). (a,b) Overviews over entire embryos. (c–f) Higher magnifications of the tail region, (c,d) lateral views, (e,f) ventral views. The morphology of cells of the median fin fold and the notochord are changed in morphants (c; arrow points to roundly shaped cell), as compared to control embryos (d). The same is true for the trunk skin (e,f). (g,h) Dorsal view on the pectoral fins. Abbreviations: epi, skin; pf, pectoral fin; tf, tail fin PHENOTYPE:
|
|
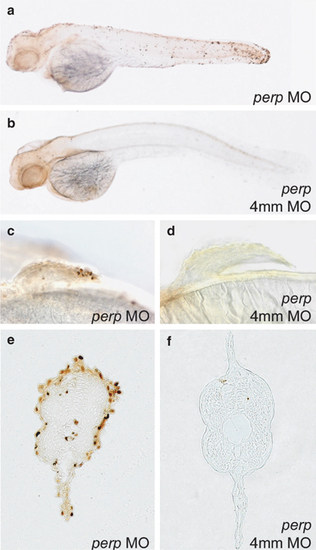
Loss of Perp causes cell death in specific embryonic tissues. TUNEL staining of embryos injected with perp MO (a, c, e), or the four mismatch control MO (4 mm MO) (b, d, f). (a–d) 48 hpf; (e, f) 36 hpf. (a,b) Whole-mount embryos; (c,d) magnified view on pectoral fins. (a) (e,f) 6 μm JB-4 transverse section through trunk at 36 hpf |
|
Loss of Perp causes caspase-dependent apoptosis. Acridine orange (a–f) and FITC-VAD-fmk (h,i) stainings of live embryos at 30 hpf; (a,c,e,h) injected with perp MO, (b) injected with perp MO and treated with caspase inhibitor zVAD-fmk, (c,f,i) injected with perp 4 mm MO, (d) uninjected control; (a–d,h,i) lateral view on tip of tail; (e,f) magnified lateral view on notochord. Note that in the notochord of perp morphants (e), all cells display altered, roundish shapes, while only some of the cells have become acridine orange positive. (g) Diagram illustrating average numbers of acridine orange-positive cells in embryos as shown in (a–d) within the tail region posterior of the anus. Number of evaluated embryos: control n=5; perp 4 mm MO-injected n=5; perp MO-injected n=6; perp 4 mm MO-injected+zVAD-FMK n=8 PHENOTYPE:
|

Unillustrated author statements EXPRESSION / LABELING:
|