- Title
-
Calcium signaling mediates proliferation of the precursor cells that give rise to the ciliated left-right organizer in the zebrafish embryo
- Authors
- Abdel-Razek, O., Marzouk, A., MacKinnon, M., Guy, E.T., Pohar, S.A., Zhushma, E., Liu, J., Sia, I., Gokey, J.J., Tay, H.G., Amack, J.D.
- Source
- Full text @ Front Mol Biosci
|
Dorsal forerunner cells give rise to the ciliated Kupffer’s vesicle that functions as the left-right organizer in the zebrafish embryo. |
|
A targeted pharmacological screen to identify pathways that mediate DFC proliferation. |
|
|
|
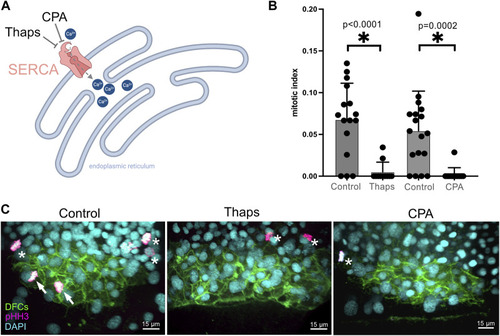
SERCA inhibitor treatments during epiboly reduce the number of ciliated KV cells and disrupt left-right patterning of the embryo. |
|
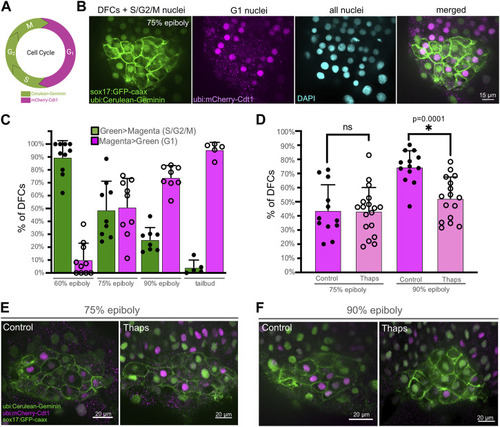
SERCA activity meditates progression of DFCs through the S/G2 phases of the cell cycle. |
|
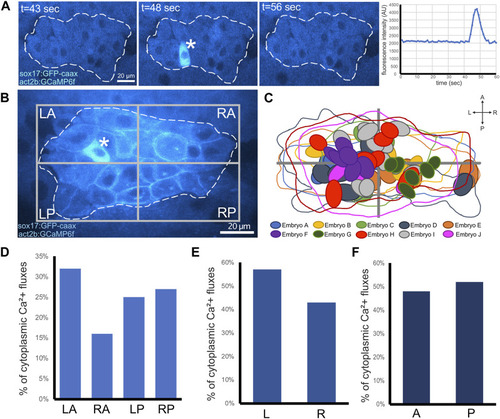
Visualization of cytoplasmic Ca2+ flux events in DFCs. |
|
Identification of nuclear Ca2+ flux in DFCs. |