- Title
-
Retina-derived signals control pace of neurogenesis in visual brain areas but not circuit assembly
- Authors
- Sherman, S., Arnold-Ammer, I., Schneider, M.W., Kawakami, K., Baier, H.
- Source
- Full text @ Nat. Commun.
|
Transcriptional profiling reveals cell types across central visual areas. |
|
HCR-FISH labeling uncovers the molecular architecture of central visual areas. Marker genes label cells in distinct anatomical regions. |
|
Cell-type diversity of central visual areas emerges in the absence of retinal input. |
|
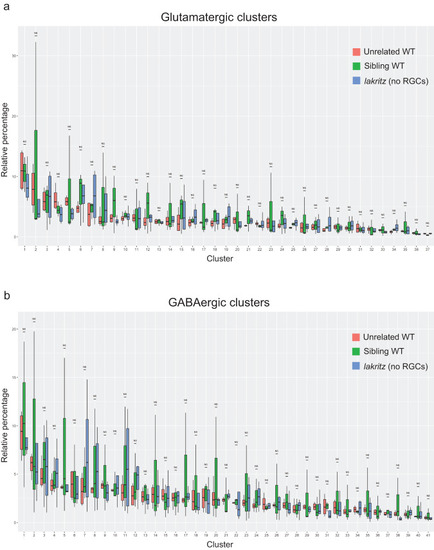
Relative cell-type proportions are largely unchanged in absence of RGCs. Bar plots showing for each cluster the variance in relative percentages across replicates ( |
|
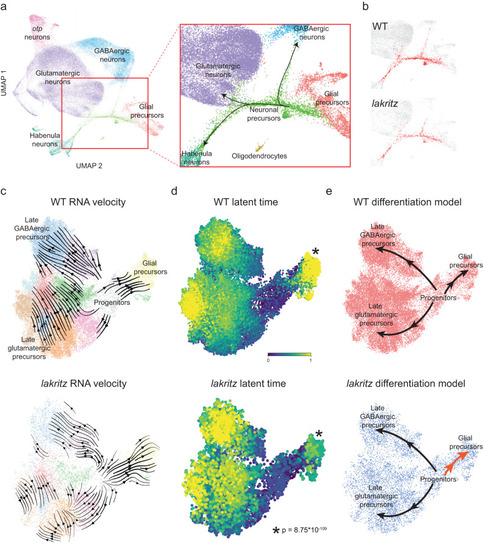
Differentiation trajectories through transcriptomic space are conserved despite absence of RGCs but differ in speed. |
|
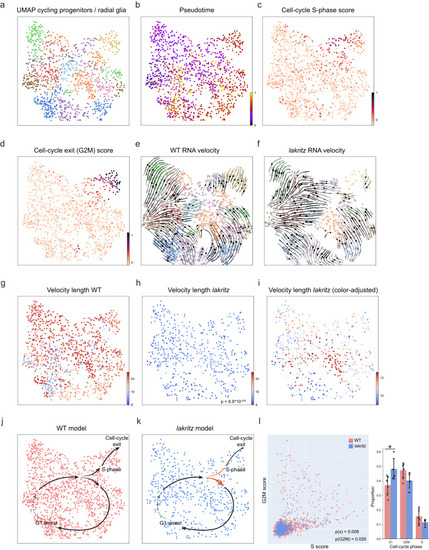
Mitotic progenitors progress more slowly through the cell cycle and exit it at a reduced rate in absence of retinal input. |
|
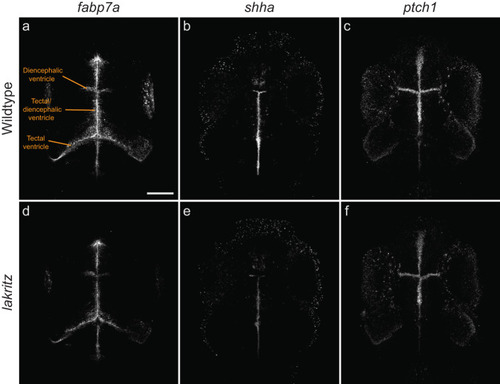
Some developmental markers are dysregulated in the ventricular zones of The zones lining the ventricles (indicated) in the midbrain and forebrain contain proliferative and differentiating cell populations. Overall patterns are unchanged in |
|
Pretectal circuitry is assembled without retinal input to drive proper behavior. |