- Title
-
Zebrafish model of RERE syndrome recapitulates key ophthalmic defects that are rescued by small molecule inhibitor of shh signaling
- Authors
- George, A., Lee, J., Liu, J., Kim, S., Brooks, B.P.
- Source
- Full text @ Dev. Dyn.
|
Zebrafish rerea homozygous mutants exhibit enlarged optic stalk and optic fissure margins that are not apposed. Brightfield images of live fish embryos (A, F), ventro-lateral angle focused on the optic stalk [OS] and histology sections of wild-type (B, C) and rerea mutant (G, H) zebrafish embryos (24 hpf) where OS (black arrow) is present on the ventral side of the eye (lateral view). Brightfield images (D, I) and histology sections of wild-type (E) and rerea mutant (J) zebrafish embryos (24 hpf) depicting optic fissure margins that are far apart (square bracket) on the ventral side of the eye (lateral view). Schematic of plane of histology sections (K). Quantification of area of the eye (L) and optic stalk (M). Distance between optic fissure margins of brightfield images (N) and histology images (O) displayed as a box and whisker plot with dots indicating individual measurements and the horizontal bar the median value. Student's t-test was applied for P values. Scale bar is 20 μm.
PHENOTYPE:
|
|
Phalloidin and DAPI stained WT (A, B) and rerea (C, D) mutant zebrafish embryos (~30 hpf) imaged from the ventral side showing optic stalk (white arrows). Table showing variability in optic stalk phenotype (E). Scale bar is 20 μm.
PHENOTYPE:
|
|
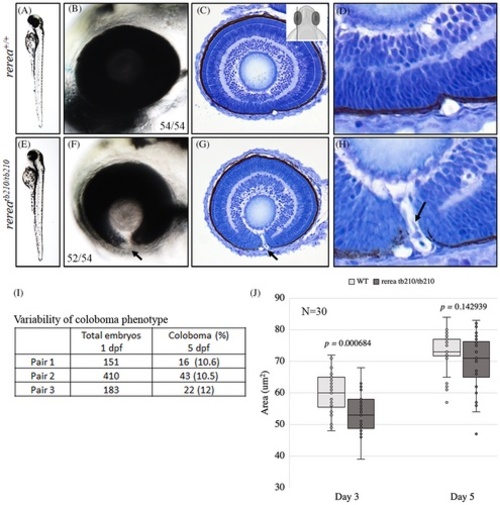
Zebrafish rerea homozygous mutants exhibit coloboma. Three-day post fertilization WT zebrafish embryos (A, B) exhibit fused optic fissure margins (54 genotyped embryos), whereas rerea homozygous mutants exhibit coloboma (E, F, 52/54 genotyped embryos). Sagittal histological sectioning of WT (C, D, N = 10 genotyped embryos) and rerea mutant (G, H, N = 10 genotyped embryos) zebrafish eye (3 dpf). The schematic in C gives the approximate plane of sectioning for the histologic sections. Coloboma showed variable penetrance in embryos at 5 dpf (I). Measurement of eye area (lateral view, Figure 2K) revealed significantly smaller eye at 3 dpf (P < .05) of rerea mutant larvae compared with WT. No statistical difference in eye size was observed at 5 dpf (J). Student's t test was used to determine level of significance and P value is provided (N = 30 genotyped embryos/group).
PHENOTYPE:
|
|
Zebrafish rerea homozygous mutants exhibit disruption of retinal optic stalk boundary. Zebrafish larvae homozygous for rerea mutation exhibit disruption of retinal optic stalk boundary (A–C, D–F), which was further confirmed by isolation of eye cups (A, D, bottom panels) and detailed coronal histological sectioning (B, E) of 6 dpf larvae. Unfused margins of optic fissure can be observed (D bottom panel, red arrow). The presence of ectopic differentiated retinal layers (C, F) was confirmed by immunostaining with ZPR-1 (photoreceptors) and HuC/D (amacrine cells and ganglion cells).
|
|
Whole mount in situ hybridization staining of zebrafish rerea homozygous mutants exhibit expanded expression domains of optic stalk markers fgf8 and pax2a. Dorsal and lateral views of 24 hpf zebrafish embryos stained with fgf8 (A–D) and pax2a (E–H) exhibited expanded expression domains (arrows) in rerea mutants (C, D, G, H) as compared with WT (A, B, E, F) embryos
|
|
Zebrafish rerea mutants exhibit disruption of proximo-distal patterning. Whole mount in situ hybridization staining of 24 hpf zebrafish embryos with vax1, aldh1a2, aldh1a3, and nlz1 probes. Numbers of embryos with patterns of staining similar to that pictured are given in each panel
|
|
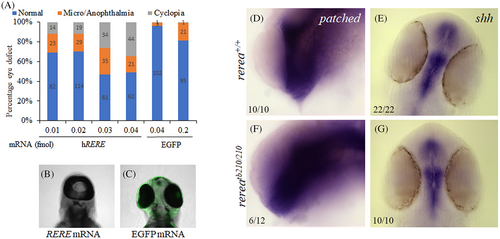
Morpholino-mediated knockdown of rerea causes coloboma. Increasing concentrations of rerea morpholino was used for knocking down rerea expression (A). Severity of coloboma is denoted by: CI-mild, CII-moderate, CIII-severe. Zebrafish embryos injected with rerea morpholino consistently exhibited coloboma at 48 hpf compared with embryos injected with control morpholino (B). Representative whole mount in situ hybridization staining with pax2a in control and rerea morpholino injected embryos 24 hpf (n ~20 embryos each) (C). WT human RERE mRNA rescues coloboma in rerea morphant fish embryos (D), this rescue was observed to be statistically significant (P < .05) for 20, 40, and 80 pg of mRNA injected, but not for 10 pg. Equal amounts of the human RERE WT and mutant (c.3466 G > A, p.Gly1156Arg) mRNA (40 pg) can rescue coloboma in rerea morphants (P < .05), but the mutant mRNA is not as efficient as WT mRNA (P < .05, E). Student's t test was used to determine level of significance and “*” denotes significant difference. NS, not significant
|
|
Injection of WT human RERE mRNA results in cyclopia. Injecting increasing concentration of human RERE mRNA results in dose-dependent increase in cyclopia phenotype (A–C). Whole mount in situ hybridization staining of 24 hpf WT and rerea mutant zebrafish embryos with patched2 (D, F) and shha (E, G) probes. Representative images with numbers of embryos showing this pattern are given in each panel.
|
|
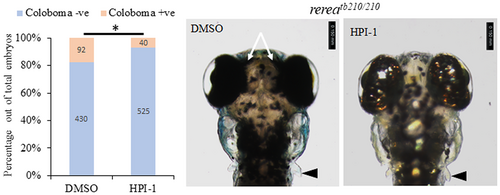
Shh signaling inhibitor HPI-1 rescues the blowout coloboma phenotype in rerea mutant zebrafish embryos. Treatment of rerea mutant zebrafish embryos with HPI-1 (5 μM) reduces the occurrence of coloboma phenotype from 71.3% to 26.3% (Student's t test, P < .05) coloboma in homozygous mutant embryos.
PHENOTYPE:
|
|
Human RERE variants exhibit hypomorphic activity in vivo and mis-localized expression in vitro. In vivo assay to determine the efficiency of human RERE variants (80 pg) to cause cyclopia in zebrafish model (A, χ2 test was used to determine level of significance and “*” denotes significant difference). In vitro assay to determine the nuclear localization pattern of human RERE wild type (WT, B) and mutant proteins (C) in HEK293 cells. Scale bar is 10 μm.
|