- Title
-
Loss of Zebrafish Mfrp Causes Nanophthalmia, Hyperopia, and Accumulation of Subretinal Macrophages
- Authors
- Collery, R.F., Volberding, P.J., Bostrom, J.R., Link, B.A., Besharse, J.C.
- Source
- Full text @ Invest. Ophthalmol. Vis. Sci.
|
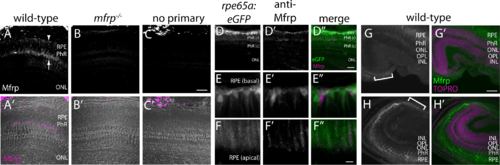
Zebrafish Mfrp is localized to the apical and basal RPE and ciliary region. (A, B, C) An anti-Mfrp antibody raised in mouse detected Mfrp in zebrafish apical and basal RPE (arrow, arrowhead, respectively). (A', B', C') Immunofluorescent signal in (A–C) is pseudocolored magenta to contrast with superimposed phase images of the cryosections. Note that rod outer segments (PhR) are found in the interval between basal and apical RPE. Cone inner and outer segments (not labeled) are in the interval between PhR and the ONL. No staining was seen when control cryosections were processed without anti-Mfrp primary antibody. Mfrp protein was seen in wild-type zebrafish RPE, but not in age-matched mutant (mfrp−/−) zebrafish with homozygous 5 bp deletions in mfrp (B, B'). Bright staining behind the eye is nonspecific staining by the secondary antibody as seen in (C). (D) Low-power image similar to (A) that includes RPE along with higher power images of basal RPE (E) and apical RPE (F) from transgenic fish expressing eGFP under control of the rpe65a promoter to delineate the RPE. Mfrp staining (D', E', F') in the RPE labels the same cells as those filled with cytoplasmic eGFP (D, E, F). Mfrp staining was seen on both apical (E') and basal (F') RPE. (D”, E”, F'') are merged images of eGFP (green) and MFRP (magenta). Note that because of the very high eGFP signal compared with that for Mfrp, (E) and (F) are single Z-slices of eGFP, whereas (E') and (F') are Z-projections through the same cells of Mfrp staining. (G, H) Mfrp staining was also seen in both the dorsal (G) and ventral (H) ciliary epithelium (brackets), contiguous with the RPE. In (G') and (H'), the Mfrp immunofluorescence is shown in green as an overlay with nuclei labeled with TO-PRO-3 (magenta). Scale bars: (A–D) 100 μm; (E–F) 20 μm; (G–H) 100 μm. PhR, photoreceptors (r, rods; c, cones); ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer.
|
|
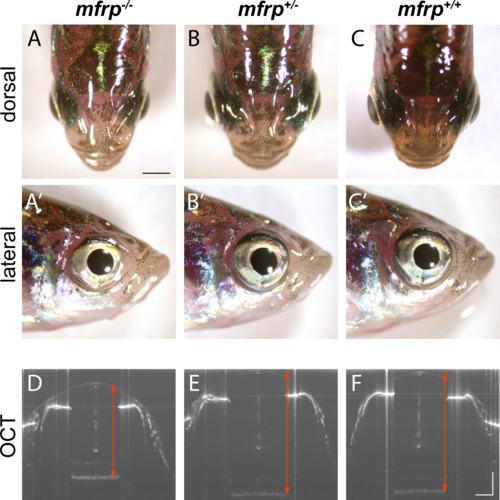
Zebrafish mfrp mutants show reduced eye size consistent with nanophthalmia. (A, B, C) Dorsal views of mfrp mutant zebrafish and sibling heterozygous and wild-type controls show mfrp homozygotes have smaller eye globes that do not project from the head as much as in their control siblings. (A', B', C') Reduced eye size is also apparent in lateral views. (D, E, F) In vivo SD-OCT imaging shows the reduced axial length (front of cornea to back of RPE; red arrows) associated with homozygous mfrp mutants. Scale bars: (A–C), 1 mm; (D–F), 300 μm.
PHENOTYPE:
|
|
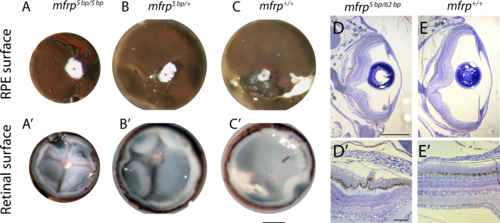
Zebrafish mfrp mutant eyes show RPE folds and compressed retinas. (A, B, C) Dissected eyecups (RPE + retina, dissected after whole-eye fixation) from mfrp mutants are smaller than sibling controls. Mutants of mfrp show folds in the RPE (red bracket), and retinas show more involutions due to compression within a smaller sclera. Optic nerves are highlighted with red asterisks. (D, E) Semithin plastic histologic sections show that mfrp mutant eyes are smaller than control siblings. RPE folds can be seen clearly at higher magnifications. Scale bars: (A–C) 1 mm; (D–E) 500 μm; (D', E') 20 μm.
PHENOTYPE:
|
|
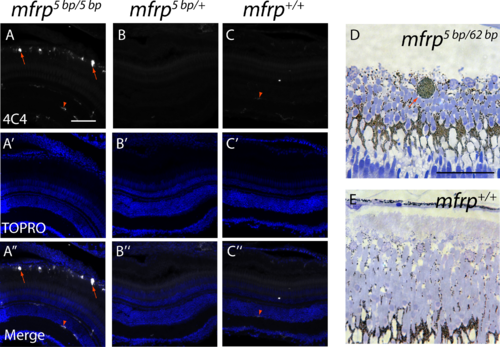
Zebrafish mfrp mutants show subretinal microglia/macrophages. (A, B, C) Cells in the subretinal space of mfrp mutant zebrafish stain positively with a microglia/macrophage marker (antibody 4C4; red arrows), whereas control heterozygous and wild-type sibling zebrafish lack such staining in the subretinal space. Resident microglia in the inner retina also stain with this marker (red arrowheads). (D, E) A pigmented subretinal cell, likely a macrophage, is visible among the photoreceptor outer segments in an mfrp mutant plastic section (red arrow). These pigmented cells are not seen in controls. Scale bars: (A–F) 100 μm; (G–H) 20 μm.
EXPRESSION / LABELING:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
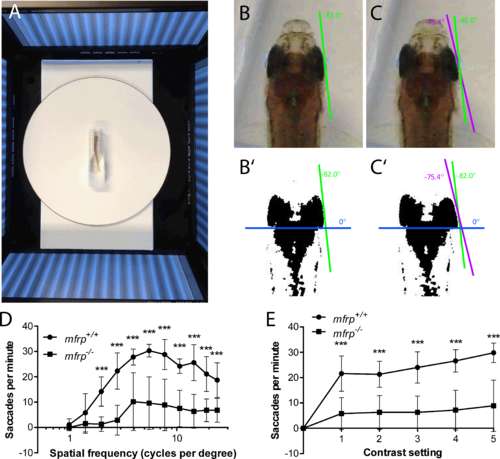
Zebrafish mutant for mfrp show reduced optokinetic response. (A) Zebrafish undergoing OKR assay are placed in a plastic cuvette at the center of a testing arena made of four computer monitors that simulate a rotating drum. (B, B', C, C') Images of zebrafish eye positions were taken immediately before (B) and after (C) a rapid eye reset following a smooth pursuit, or saccade. Images were thresholded in ImageJ (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) to highlight the highly melanized posterior part of the eye used to define eye position. (D) Fish that were mfrp−/− showed lower visual acuity than mfrp+/+ siblings at 12 mpf by OKR assay. (E) Fish that were mfrp−/− showed fewer saccades per minute than mfrp+/+ siblings by OKR assay when contrast settings were reduced; however, this appeared to be due to reduced visual acuity rather than lack of contrast sensitivity. Graphs show mean saccades per minute ± SD.
PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
|
|
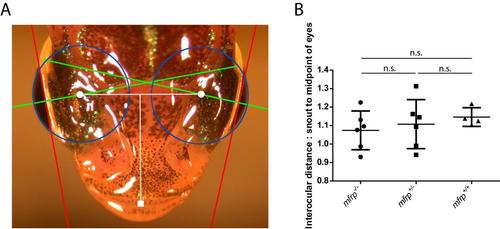
Zebrafish mfrp mutants do not have shorter interocular distances than wild-type sibling controls. A. Zebrafish axial lengths were measured by SD-OCT and the same fish were imaged dorsally. Axial lengths (green) were used to calculate eye globe circumferences (blue) and geometric centers of eyes (white circles). The distance between centers of eyes was measured and normalized against the distance from the tip of the snout (white square) to the midpoint of the eyes to control for different overall sizes of fish. B. mfrp mutants did not show significantly different interocular distance:snout to midpoint of eyes ratio when compared to wild-type controls. Six fish were measured for mfrp+/+ and mfrp+/- fish, while 4 fish were measured for mfrp-/-. |
|
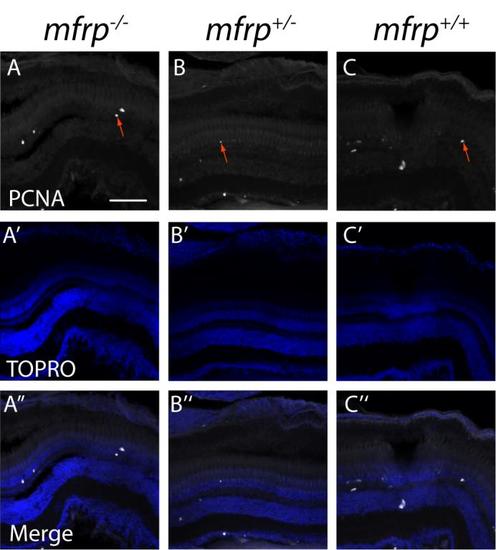
Zebrafish mfrp mutant eyes do not show increases in the cell proliferation marker, PCNA. mfrp mutant retinas (A) have similar numbers of cells that stain for proliferating cell nucleic antigen (PCNA) when compared to heterozygotes (B) or wild-type (C) controls. PCNA-positive cells are indicated by red arrows). A'-C'. TOPRO images. A''-C''. Merged images. Scale bar: 100 μm. |