- Title
-
CaMK-II activation is essential for zebrafish inner ear development and acts through Delta-Notch signaling
- Authors
- Rothschild, S.C., Lahvic, J., Francescatto, L., McLeod, J.J., Burgess, S.M., and Tombes, R.M.
- Source
- Full text @ Dev. Biol.
|
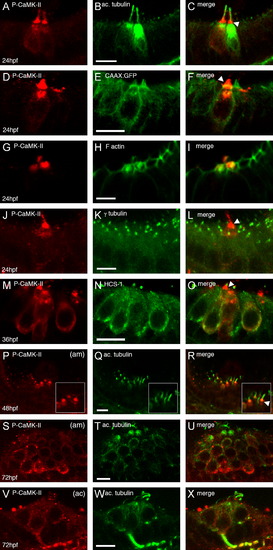
Localization of activated CaMK-II in 24–72 hpf zebrafish embryonic ears. Embryos were immunostained for P-CaMK-II (A, D, G, J, M, P, S, V) in wild-type or CAAX:GFP embryos and then counterstained for acetylated tubulin (B, Q, T, W), F actin (H), γ tubulin (K) or HCS-1 (N). Arrowheads point to the most intense P-CaMK-II staining. Images were acquired laterally with anterior to the left at 24 hpf (A–L), 36 hpf (M–O), 48hpf (P–R) or 72 hpf (S–X). am=anterior macula, ac=anterior crista. All scale bars=5 μm. EXPRESSION / LABELING:
|
|
Otolith formation is defective in camk2g1 morphants. Gross morphology of otoliths was assessed at 30 hpf (A–C) and 72 hpf (D–F) in embryos injected with 1 ng control MO (A, D) or 1 ng camk2g1 MO (B, C, E, F). Scale bar (50 μm) in A applies to A–C and in D applies to D–F. Defects in otolith shape were quantitated at 72 hpf for 186 morphant embryos and compared to 118 control embryos (G). P-CaMK-II (red) at the base of the kinocilia in 24 hpf control (H, H′) embryos (arrowhead) was lost in camk2g1 morphants (I, I′). Cilia were counterstained with anti-acetylated tubulin (green) with anterior on the left. Scale bars in H, I=5 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
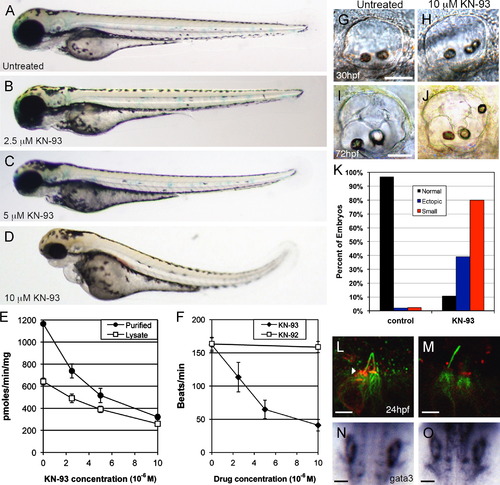
Effects of KN-93 on embryonic development. Gross morphology was assessed at 72 hpf in untreated embryos (A) and in embryos continuously treated from cleavage stage with 2.5 μM KN-93 (B), 5 μM KN-93 (C) and 10 μM KN-93 (D). CaMK-II kinase activity assays were conducted on 3 dpf whole embryo lysates and 3dpf partially purified CaMK-II in the presence of increasing concentrations of KN-93 (E). Heart rates at 72 hpf were averaged from at least 20 embryos per drug concentration (F). 10 μM KN-93 treatment resulted in otolith defects at 30 hpf (G, H) and 72 hpf (I, J). Scale bar (50 μm) in G applies to G, H and in I applies to I, J. Otolith defects assessed at 72 hpf were tabulated for 125 control and 182 10 μM KN-93 treated embryos (K). P-CaMK-II (red) was diminished in 10 μM KN-93 (M) treated embryos compared to control embryos (L, arrowhead) at 24 hpf but gata3 was expressed in the otic vesicles of both control (N) and 10 μM KN-93 (O) treated 24 hpf embryos. Scale bars=5 μm (L, M) and 50 μm (N, O). EXPRESSION / LABELING:
PHENOTYPE:
|
|
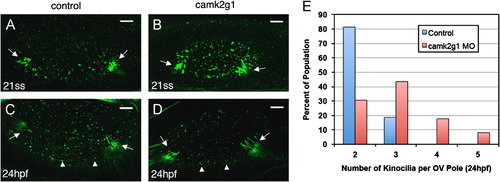
Motile cilia and kinocilia form in camk2g1 morphants. Control (A, D) and camk2g1 morphants (B, D) were labeled with anti-acetylated tubulin (green) at 21ss and at 24 hpf. Kinocilia formed in the proper locations (arrows) emerging from hair cells at the anterior and posterior poles of the otic vesicle. Anterior is to the left. Scale bars=5 μm. A histogram of the number of kinocilia per otic vesicle (OV) pole at 24 hpf (E) revealed a shift in morphant embryos (n=43) to higher values when compared to control embryos (n=62). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Formation of maculae and cristae in camk2g1 morphants. Control (A, C, D) and camk2g1 morphants (B, E, F) at 72 hpf were stained for F-actin (green) and acetylated tubulin (red). Sensory patches include anterior macula (am), posterior macula (pm), anterior crista (ac), lateral crista (lc) and posterior crista (pc). The posterior macula is out of view in the control image. Anterior is to the left. Scale bars=20 μm (A, B) and 5 μm (C–F). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Formation of maculae and cristae in camk2g1 morphants. Control (A, C, D) and camk2g1 morphants (B, E, F) at 72 hpf were stained for F-actin (green) and acetylated tubulin (red). Sensory patches include anterior macula (am), posterior macula (pm), anterior crista (ac), lateral crista (lc) and posterior crista (pc). The posterior macula is out of view in the control image. Anterior is to the left. Scale bars=20 μm (A, B) and 5 μm (C–F). |
|
Model of action. (A) Screen shots from control and camk2g1 morphant embryos at 24 hpf. Control embryos have two immotile kinocilia (white arrows) bound to the seeding otolith. Camkg1 morphants have extra hair cells as demonstrated by extra kinocilia (B, white arrows). (B) The additional hair cells are formed as a result of improper Delta–Notch signaling. (1) In control embryos CaMK-II aids in secretory vesicle trafficking and fusion to the plasma membrane. (2) Once Delta is deposited at the membrane it can bind to the Notch receptor. (3) Transendocytosis occurs due to mib (mindbomb; E3 ubiquitin ligase) activity, causing the NICD to be cleaved and enter into the nucleus influencing transcription. In the absence of CaMK-II, Delta ligands are not deposited at the membrane and accumulate in secretory vesicles in the cytosol, therefore inhibiting transcription in the Notch expressing cell. |
|
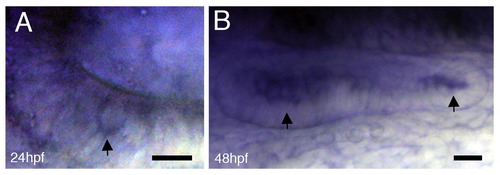
Camk2g1is expressed in the zebrafish inner ear.Camk2g1 expression occurs in the otic vesicle at 24 hpf (A) and 48 hpf (B) in the hair cells (arrows). Anterior is to the left. Scale bars=5 μm. |
|
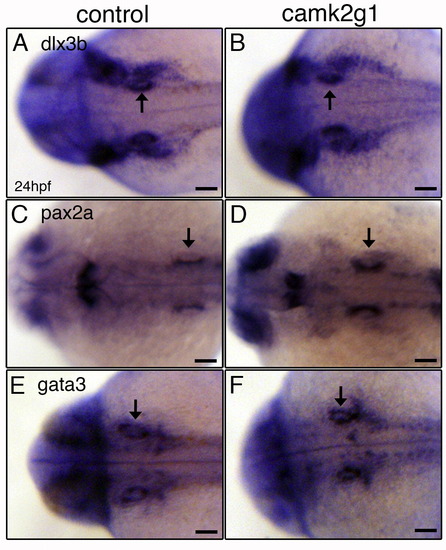
Inner ear precursors are specified properly incamk2g1morphants. Control and camk2g1 morphants were assayed by in situ hybridization for expression of dlx3 (A, B, lateral view), pax2 (C, D, dorsal view) and gata3 (E, F, dorsal view) in the ear at 24 hpf. Arrows point to the otic vesicle. Anterior is to the left. Scale bars=50 μm. |
Reprinted from Developmental Biology, 381(1), Rothschild, S.C., Lahvic, J., Francescatto, L., McLeod, J.J., Burgess, S.M., and Tombes, R.M., CaMK-II activation is essential for zebrafish inner ear development and acts through Delta-Notch signaling, 179-88, Copyright (2013) with permission from Elsevier. Full text @ Dev. Biol.