- Title
-
Both STING and MAVS Fish Orthologs Contribute to the Induction of Interferon Mediated by RIG-I
- Authors
- Biacchesi, S., Merour, E., Lamoureux, A., Bernard, J., and Bremont, M.
- Source
- Full text @ PLoS One
|
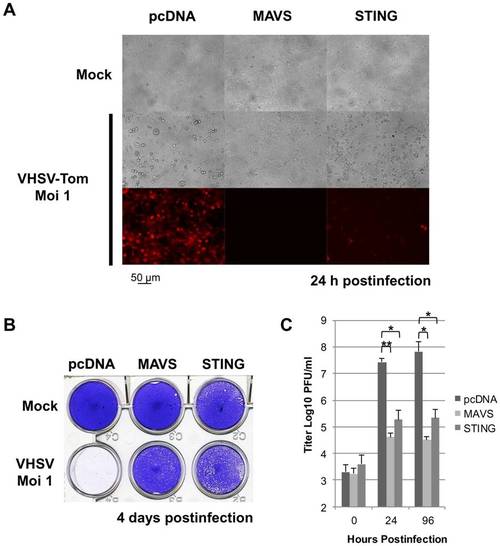
Zebrafish STING is a strong antiviral protein. EPC cells were transfected with 2 μg of pcDNA-STING, and pcDNA-MAVS or an empty vector (pcDNA) as positive and negative controls, respectively. At 48 h posttransfection, EPC cells were infected with a recombinant rVHSV-Tom expressing the tdTomato fluorescent protein at an MOI of 1 and incubated at 15°C. Cell monolayers were visualized under a UV-visible light microscope at 24 h postinfection (A) and then stained with crystal violet 4 days postinfection (B). The culture supernatants from cells infected with rVHSV-Tom were collected at 0, 24 and 96 h postinfection and the viral titer was determined by plaque assay on EPC cells (C). Each time point was represented by three independent experiments, and each virus titration was done in duplicate. Means are shown. The standard errors were calculated and the error bars are shown. Asterisks indicate significant difference (*p<0.01 and **p<0.001) as determined by Student′s t test. |
|
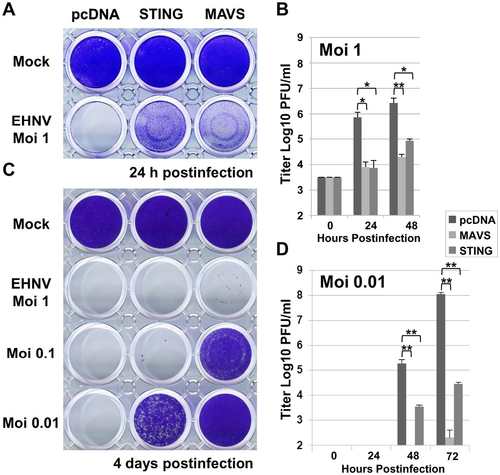
Overexpression of STING induces an antiviral immunity against a DNA virus. EPC cells were transfected with 2 µg of pcDNA-STING, and pcDNA-MAVS or an empty vector (pcDNA) as positive and negative controls, respectively. At 48 h posttransfection, EPC cells were infected with a DNA virus of the Iridoviridae family, i.e., EHNV, at MOI of 1, 0.1 and 0.01 (A and C). The culture supernatants were collected at 0, 24, 48 and 72 h postinfection and the viral titer was determined by plaque assay on EPC cells (B and D). Cell monolayers were then stained with crystal violet either at 24 h postinfection (A) or 4 days postinfection (C) depending to the MOI used, as indicated. Each time point was represented by three independent experiments, and each virus titration was done in duplicate. Means are shown together with the standard errors. Asterisks indicate significant difference (*p<0.01 and **p<0.001) as determined by Student’s t test. |
|
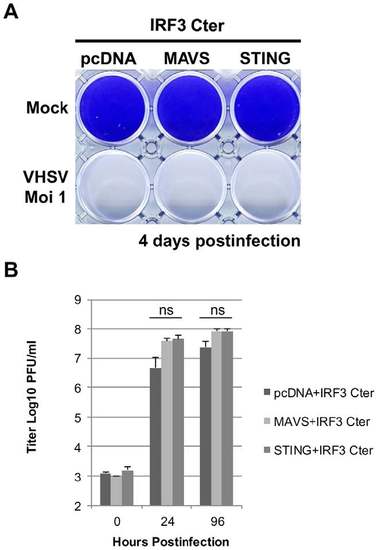
STING antiviral response is mediated by IRF3. EPC cells were co-transfected with 2 µg of pcDNA-IRF3-Cter encoding a dominant-negative mutant of IRF3, and 2 µg of pcDNA-STING, pcDNA-MAVS or an empty vector. At 48 h posttransfection, EPC cells were infected with a recombinant rVHSV-Tom expressing the tdTomato fluorescent protein at an MOI of 1 and incubated at 15°C. The culture supernatants were collected at 0, 24 and 96 h postinfection and the viral titer was determined by plaque assay on EPC cells. Cell monolayers were then stained with crystal violet either at 96 h postinfection. Each time point was represented by three independent experiments, and each virus titration was done in duplicate. Means are shown together with the standard errors. “ns” indicate non-significant difference (p>0.05) as determined by Student’s t test. |
|
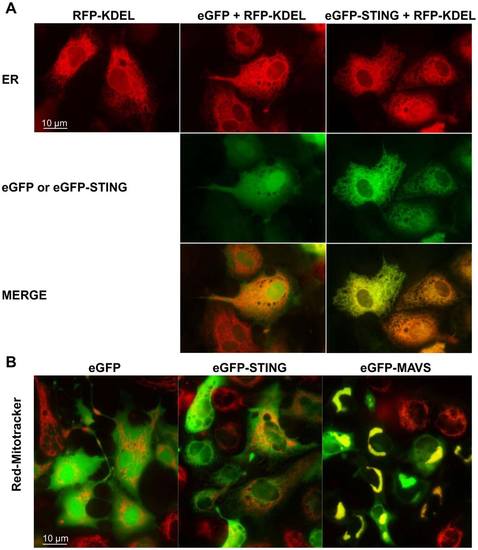
Localization of zebrafish STING to endoplasmic reticulum. peGFP-STING or peGFP-MAVS and peGFP, as negative controls, were transfected together with a plasmid encoding the Red Fluorescent protein fused to a reticulum endoplasmic location signal (RFP-KDEL) into EPC cells (A). The mitochondria were in vivo stained with a red MitoTracker (B). The cells were imaged by microscopy 24 h post-transfection. The yellow staining in the overlay image indicates colocalization of STING and RFP-KDEL (A) or MAVS and MitoTracker (B). |
|
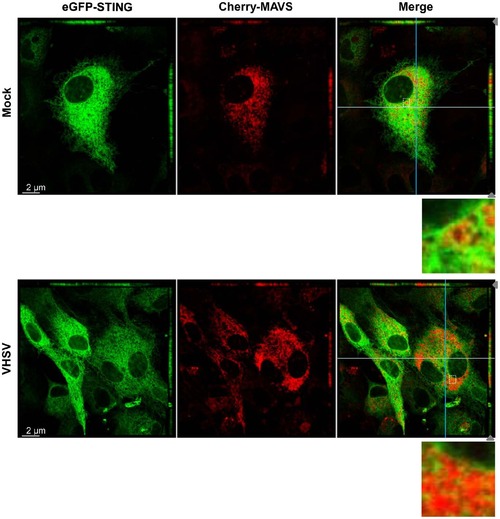
STING and MAVS are in close vicinity in mitochondrial-ER contact regions. EPC cells were cotransfected with 1 µg of peGFP-STING and 1 µg of pCherry-MAVS. At 48 h posttransfection, EPC were infected with VHSV at an MOI of 1 and incubated at 15°C for 24 h before imaging by confocal microscopy. For both panels the main images show a section of the cell monolayer in the xy plane at the z position indicated by the grey arrow head in the xz plane (small top panel) and the yz plane (small right side panel). Orthogonal projections of confocal sections shown in the top and right side panels are in the cutting plane indicated by the white and the blue lines corresponding to the xz and yz planes, respectively. Zoomed insets of boxed areas in merged images are also presented. |