- Title
-
Wnt/β-catenin signaling directly regulates Foxj1 expression and ciliogenesis in zebrafish Kupffer’s vesicle
- Authors
- Caron, A., Xu, X., and Lin, X.
- Source
- Full text @ Development
|
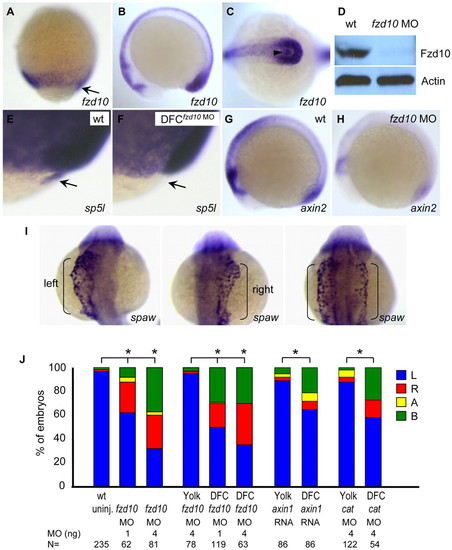
Wnt/β-catenin signaling regulates LR asymmetry KV cell-autonomously. (A-C) fzd10 expression pattern. Shown are a lateral view of 80% epiboly (A), and lateral (B) and dorsal (C) views of the tail bud region of 10-somite staged zebrafish embryos with anterior to the left. Arrow, DFCs; arrowhead, KV. (D) Western blot showing diminished Fzd10 level in fzd10 morphants. Actin was used as a loading control. (E-H) fzd10 transduces Wnt/β-catenin signaling. DFC-specific injection of fzd10 MO depleted sp5l expression from DFCs (F). Knockdown of Fzd10 resulted in downregulation of axin2 expression (H). Shown are lateral views of 80% epiboly (E,F) and 10-somite (G,H) staged embryos. Arrow indicates DFCs. (I) Representative images of spaw expression (bracket) in DFCfzd10 MO embryos at the 21-somite stage. (J) DFC/KV-specific reduction of Wnt/β-catenin signaling randomizes spaw expression. Percentages of spaw expression were determined in 21-somite staged embryos. MO (ng), amount of MO used; N, number of embryos examined. L, left-side; R, right-side; A, absence; B, bilateral. *P<0.01 compared with corresponding modulations in the yolk only or with uninjected controls. EXPRESSION / LABELING:
|
|
Reduction of Wnt signaling results in shorter and fewer cilia in KV. (A-F) Dose-dependent effect of Wnt signaling on cilia length and number. (A-C) Tg(hsp:dkk1-GFP) fish were bred with wild-type fish. Their progenies were heat shocked at 60% epiboly for 30 minutes (B) or 60 minutes (C) to induce Dkk1 expression. Moderate induction of Dkk1 (Dkk+) resulted in shorter cilia (B) whereas higher level of Dkk1 induction (Dkk++) led to shorter and fewer cilia (C). (D-F) DFC-targeted injection of 1 ng of fzd10 MO reduced cilia length, but not number (E); 4 ng of MO reduced both cilia length and number (F). Cilia were visualized by anti-acetylated tubulin antibody staining of 10-somite staged embryos. Scale bars: 5 μm. (G-I) Wnt signaling is essential for dnah9 expression in DFCs. Dkk1 induction (H) or DFC-targeted injection of β-catenin1 MO (I) downregulated dnah9 expression. Shown are ventral views of 95% epiboly staged embryos. (J,K) Quantification of cilia length (J) and number (K) in 10-somite staged embryos. Approximately 12-16 embryos were analyzed for each group. Tg(hsp:β-catenin-GFP) fish were bred with wild-type fish. Their progenies were heat shocked at 60% epiboly for 60 minutes to induce β-catenin1 (Cat++) expression. Data are represented as mean±s.d. *P<0.01. NS, not statistically significant. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Wnt signaling regulates KV ciliogenesis through modulation of foxj1a expression. (A-D) Wnt signaling is required for foxj1a expression in DFCs. foxj1a expression in DFCs was severely downregulated in Dkk1-expressing zebrafish embryos (B; 37/38) and embryos injected with fzd10 MO into DFCs (C; 24/27) compared with wild-type controls (A). Induction of β-catenin1 did not appear to affect foxj1a expression (D). (E-H) Ectopic expression of foxj1a restores dnah9 levels in embryos with impaired Wnt activity. Downregulated dnah9 expression in DFCfzd10 MO embryos (F; 16/18) was enhanced by DFC-targeted foxj1a overexpression (G; 15/19). Overexpression of foxj1a RNA alone did not significantly alter dnah9 level (H). Shown are ventral views of 90-95% epiboly staged embryos. (I,J) Ectopic expression of foxj1a partially rescues KV cilia length (I) and, to a lesser extent, cilia number (J). Approximately 10-18 embryos for each group were analyzed at the 10-somite stage. Data are represented as mean±s.d. *P<0.01; #P<0.05. NS, not significant (P>0.05). Tg(hsp:dkk1-GFP) and Tg(hsp:β-catenin-GFP) embryos were heat shocked at 50% epiboly for 60 minutes. (K) Ectopic expression of foxj1a partially restores left-sided spaw expression in embryos with impaired Wnt activity. Percentages of spaw expression were determined in 18-to 21-somite staged embryos. N, number of embryos examined. L, left-side; R, right-side; A, absence; B, bilateral. EXPRESSION / LABELING:
|
|
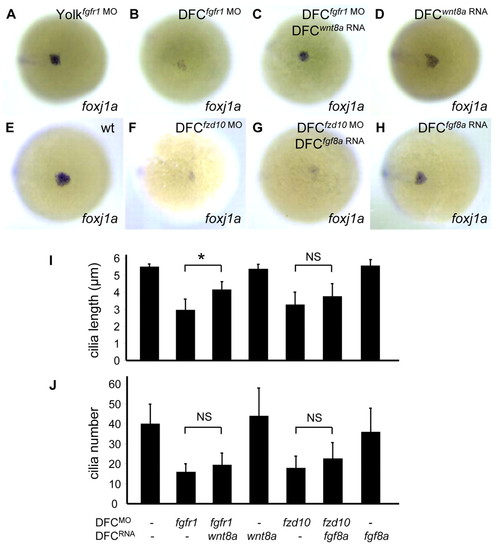
Wnt signaling functions downstream of the FGF pathway in regulation of foxj1a expression and cilia formation. (A-D) Ectopic expression of Wnt ligands restores foxj1a levels in embryos with reduced FGF signaling. DFCfgfr1 MO embryos exhibited downregulated foxj1a expression in DFCs (B; 17/18) compared with controls (A). Co-injection of wnt8a RNA with fgfr1 MO restored foxj1a expression (C; 16/19). Ectopic expression of wnt8a RNA alone had no apparent effect on foxj1a expression (D). (E-H) Ectopic expression of FGF ligands fails to restore foxj1a expression in embryos with reduced Wnt signaling. Downregulated foxj1a expression in DFCfzd10 MO embryos (F) was not restored by co-injection of fgf8a RNA (G). Ectopic expression of fgf8a RNA alone had no apparent effect on foxj1a expression (H). Shown are ventral views of 90-95% epiboly staged embryos. (I,J) Ectopic expression of Wnt ligands partially rescues cilia length defects in embryos with reduced FGF signaling. Shown are quantification of cilia length (I) and cilia number (J). Approximately 12-20 embryos were analyzed for each group. Data are represented as mean±s.d. *P<0.01. NS, not significant (P>0.05). EXPRESSION / LABELING:
|
|
Wnt signaling directly regulates foxj1a transcription. (A) Schematic of foxj1a enhancers. Sequence of foxj1a that is located approximately –6.2 kb to –4.6 kb upstream of the ATG start codon was used to generate a series of report constructs. Blue bar, conserved sequence between zebrafish and tetradon. Hatched bar, predicted first non-coding exon. Red line, putative Lef1/Tcf binding site. These fragments were inserted in front of the gfp sequence in a tol2 vector, and the resulting plasmids were co-injected with tol2 transposase RNA into embryos. Fluorescent GFP signals in KV were scored. (B,C) A 0.6 kb enhancer sequence contains Wnt-responsive cis-acting elements. Transgenic Tg(0.6foxj1a:gfp) embryos showed reporter gfp expression in DFCs (B; 12/12). DFC-targeted injection of fzd10 MO abolished gfp expression in DFCs (C; 14/15). Shown is in situ hybridization using gfp as a probe in a ventral view of 95% epiboly staged embryos. Dashed circle indicates DFC region. (D-I) Putative Lef1/Tcf binding sites are required for foxj1a expression in KV. GFP reporter expression in stable transgenic Tg(0.6foxj1a:gfp) embryos recapitulated foxj1a expression pattern in KV (D,E), PDs (E,F) and FP (E,F). After all three putative Lef1/Tcf binding sites in the 0.6 kb enhancer were deleted using a site-directed mutagenesis kit, stable transgenic Tg(0.6Δfoxj1a:gfp) embryos lacked GFP expression in KV (G,H) and PDs (H,I) but maintained GFP expression in FP (H,I). Arrow indicates KV, open arrowhead PDs and filled arrowhead FP. Shown are embryos at 10-12 somites (D,G), 16 somites (E,H) and 30 hpf (F,I). EXPRESSION / LABELING:
|
|
Dkk1 induction impairs ciliogenesis in PD and OV. (A-D) Induction of Dkk1 downregulates foxj1 expression in PDs and OVs. foxj1a level in PDs was downregulated in transgenic Dkk1 embryos (B; 8/8) versus non-transgenic siblings (A). foxj1a expression in FP was not affected (B). foxj1b expression in the otic placode was downregulated in transgenic Dkk1 embryos (D; 8/8) versus non-transgenic siblings (C). Arrow indicates PDs, asterisk FP, and arrowhead the otic placode. (E,F) Induction of Dkk1 suppressed dnah9 expression in PDs and OVs (F; 11/11) compared with controls (E). White arrow represents PDs, and white arrowhead the otic placode. Shown are dorsal views (A,B) and lateral views (C-F) of 10-somite staged embryos with anterior to the left. (G-J) Induction of Dkk1 results in shorter and fewer cilia in PDs and OVs. Cilia were visualized by anti-tubulin antibody staining of 26-somite staged embryos. White arrow indicates tethering cilia in OVs. (K,L) Quantification of cilia length in PDs (K) and OVs (tethering cilia) (L). Data are represented as mean±s.d. Approximately 9-18 embryos were used for each group. *P<0.01. (M-O) Induction of Dkk1 results in kidney cysts and otolith malformation. Cystic distension of PD (arrow in M; 45/73) was observed at 24 hpf. One otolith (arrowhead in O; 89/95) was seen at 2 dpf. Tg(hsp:dkk1-GFP) embryos were heat activated at 60% epiboly. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Generation of functional Tg(hsp:dkk1-GFP) and Tg(hsp:β-catenin-GFP) transgenic fish. (A) Schematic of hsp70 promoter-driven dkk1 expression. An upstream sequence (<1.6 kb) of zebrafish hsp70-4 (Halloran et al., 2000) and full-length zebrafish dkk1 encoding sequence were inserted in-frame into the tol2 GFP vector. The resulting plasmid was co-injected with tol2 transposase RNA into one-cell staged embryos. (B) The fused GFP tag allows discrimination of Dkk1-expressing embryos (GFP+) from non-transgenic siblings (GFP-) after heat-shock treatment. Transgenic Dkk1 embryos that were heat activated at 30% epiboly had tail/trunk truncation, a characteristic phenotype of loss of Wnt signaling (Lekven et al., 2001). Heat-shocked non-transgenic siblings exhibited normal morphology. (C) Representative images of pitx2 expression in transgenic Dkk1 embryos (Dkk+) heat-shocked at the bud stage. Shown are dorsal views of 22-somite staged embryos. Normal left-sided expression became randomized. (D) Schematic of hsp70 promoter-driven β-catenin1 expression. An upstream sequence (<1.6 kb) of zebrafish hsp70-4 (Halloran et al., 2000) and full-length zebrafish β-catenin1 encoding sequence were inserted in-frame into the tol2 GFP vector. (E) Transgenic β-catenin1 embryos activated at the sphere stage had anterior head truncation, a characteristic phenotype of gain of Wnt signaling (Lekven et al., 2001). (F) Induction of β-catenin1 (Catenin+) at the sphere stage abolished the expression of forebrain marker foxg1 but maintained the expression of midbrain-hindbrain boundary marker her5 compared with heat-shocked non-transgenic siblings (Catenin-). Arrows indicate foxg1 expression. |
|
Genes participating in the Wnt/β-catenin pathway are expressed in KV. (A) charon expression in KV. (B-H) Components of the Wnt/β-catenin pathway are expressed within or near KV. Shown are lateral views (A-C) and dorsal views (D-H) of the tail bud region of 10-somite staged embryos. (I-N) Two-color fluorescence in situ hybridization showed a partial overlapping of wnt3a or wnt8a (red) and charon (green). Shown are the tail bud regions of 10-somite staged embryos. (O-R) Fzd10 is not part of the Wnt/PCP pathway. Injection of 4 ng of fzd10 MO into one-cell staged embryos did not elicit defects in somite formation (P) and convergent extension movement as revealed by ntl, hgg1 and dlx3 in situ staining (R). |
|
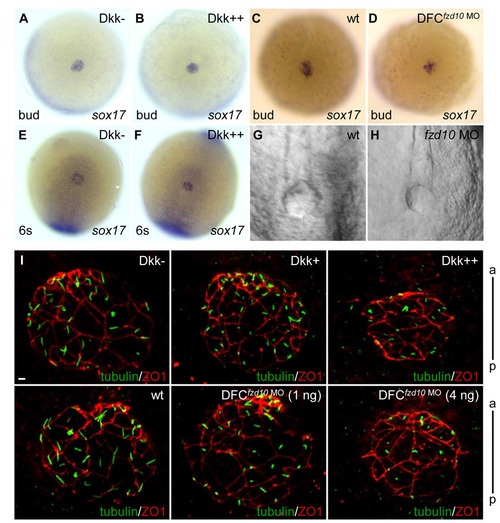
Wnt signaling is not required for DFC specification, KV formation or apical-basal polarization of KV cells. (A-D) DFC specification was assessed by sox17 levels at the bud stage. Induction of Dkk1 (B) or DFC-targeted injection of 4 ng of fzd10 MO (D) did not alter sox17 expression compared with controls (A,C). (E,F) KV cell maintenance was evaluated by sox17 levels at 6 somites. Induction of Dkk1 (F) did not affect sox17 expression relative to controls (E). (G,H) KV formation was normal in embryos injected with 4 ng of fzd10 MO (H) compared with controls (G). Shown are ventral (A-D) and dorsal (E-H) views of the tail bud region. (I) Activation of Dkk1 or DFC-targeted injection of fzd10 MO had no effect on the expression of ZO1 (an apical marker of KV cells). Shown are the tail bud region immunostained using antibodies against acetylated tubulin (green) and ZO1 (red) at 10 somites. Tg(hsp:dkk1-GFP) embryos were heat shocked at 60% epiboly for 30 minutes (Dkk+) or 60 minutes (Dkk++) to induce Dkk1 expression. Scale bar: 5 μm. a, anterior region of KV; p, posterior region of KV. |
|
FGF signaling regulates foxj1a expression and cilia length and number in a dose-dependent manner. (A-C) foxj1a expression was downregulated in DFCs in embryos incubated with 10 μm of SU5402 (B) and further reduced in embryos incubated with 20 μm of SU5402 (C). Shown are dorsal views of 90% epiboly staged embryos. (D-F) DFC-targeted injection of fgfr1 MO inhibited foxj1a expression dose dependently. (G-I) DFC-targeted injection of fgfr1 MO did not affect DFC specification as revealed by normal sox17 expression. (J,K) Quantification of KV cilia length and number. SU5402 was incubated with embryos at 60% epiboly stage for 20 minutes. *P<0.01. Approximately 12-20 embryos at 10 somites were examined for each group. Error bars represent s.d. |
|
Wnt signaling does not require intermediate protein synthesis to modulate foxj1a expression. (A,B) Induction of Dkk1 inhibited foxj1a expression rapidly. (C,D) Induction of β-catenin1 enhanced foxj1a expression rapidly. Tg(hsp:dkk1-GFP) and Tg(hsp:β-catenin-GFP) embryos were heat shocked at 30% epiboly for 30 minutes, and collected for in situ staining 30 minutes later. Shown are dorsal views of 50% epiboly staged embryos. |
|
Wnt signaling is not required for specification of developing PDs and OVs. (A-F) Transgenic Dkk1 embryos had nearly normal pax2 expression in PDs (B,D) and OVs (B,F) compared with non-transgenic siblings (A,C,E). Tg(hsp:dkk1-GFP) embryos were heat-shocked at 60% epiboly for 60 minutes and collected at the 10-somite stage for in situ staining. Shown are lateral views (A,B) and dorsal views (C-F) with anterior to the left. Arrow indicates PDs, and arrowhead OVs. |