- Title
-
Tissue-specific compensatory mechanisms maintain tissue architecture and body size independent of cell size in polyploid zebrafish
- Authors
- Small, C.D., Benfey, T.J., Crawford, B.D.
- Source
- Full text @ Dev. Biol.
|
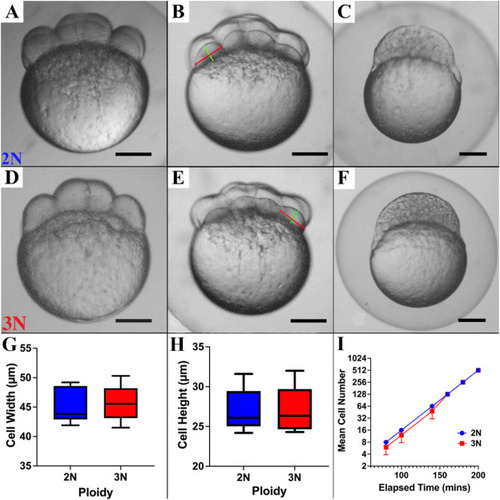
Cell cycle and cell size are not affected by ploidy in pre-gastrula zebrafish embryos. Diploids (A–C) and triploids (D–F) are morphologically indistinguishable at the 8-cell (A and D), 16-cell (B and E) and 512-cell (C and F) stages. A-F) Bright-field micrographs of pre-gastrula stage diploid and triploid zebrafish embryos (scale bar = 50 μm). G) Cell width and H) height [red and green lines, respectively, in B and E] are not significantly affected by ploidy (2 blastomeres per embryo, n = 8 embryos per ploidy, p = 0.81 and 0.95, respectively). I) Mean cell number during the first 200 min of development is not significantly affected by ploidy (n = 8 embryos per ploidy, p = 0.23). |
|
Erythrocytes are larger but fewer in number in triploid zebrafish embryos. A + C) DIC micrographs of embryonic erythrocytes in the dorsal aorta at 48 hpf in a diploid (A) and a triploid (C) embryo. B + D) DIC micrographs of embryonic erythrocytes traversing an ISV at 48 hpf in a diploid (B) and triploid (D). E) Triploid erythrocytes are larger than diploid erythrocytes in the dorsal aorta (n = 8 embryos per ploidy [30 cells per embryo], p < 0.001) and F) in the ISVs (n = 8 embryos per ploidy [5–8 cells per embryo], p < 0.001). G) Triploid erythrocytes deform relatively more than diploid cells when traversing ISVs (8 embryos per ploidy, p < 0.01). H) Triploid embryos have fewer circulating erythrocytes (n = 8 embryos per ploidy, p < 0.001). |
|
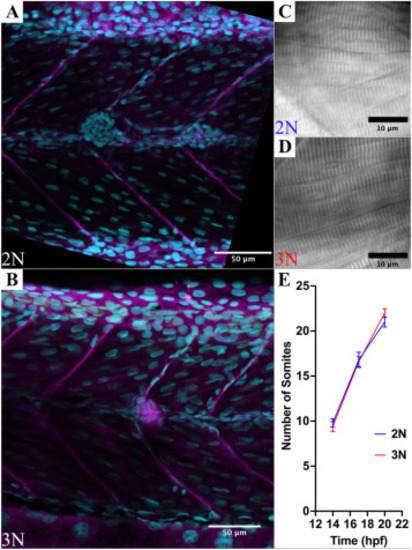
Ploidy does not affect myotome size, sarcomere size, or the rate of somitogenesis in zebrafish embryos. A + B) Confocal micrographs of anti-laminin immunostains (magenta channel) co-stained with Draq5 (cyan channel) of diploid (A) and triploid (B) myotomes. Myotome size was not significantly different along the anteroposterior axis or the dorsoventral axis (n = 5 embryos per ploidy, p = 0.18, and p = 0.99 respectively). C + D) DIC micrographs of diploid and triploid myofibers. Sarcomere size was not significantly affected by ploidy (n = 8 embryos per ploidy, p = 1). E) The rate of somitogenesis is not affected by ploidy (n = 8 embryos per ploidy, p = 0.22). |
|
The size and number of myofibers in each myotome are not affected by ploidy. A + B) Confocal micrographs of diploid (A) and triploid (B) myotomes with actin labeled with phalloidin-Alexa568 (red channel) and DNA stained with DAPI (cyan channel). Scale bars are 100 μm. C + D) Single focal plane images from confocal stacks from A + B resliced along the AP axis (dorsal to the left). E) The number of myofibers in a diploid myotome (C) is not significantly different than the number in a triploid myotome (D) (n = 3 embryos per ploidy, p = 0.065). |
|
The number of nuclei per myofiber is significantly reduced in triploid embryos. A + B) Confocal projections of myotomes in diploid (A) and triploid (B) Tg (h2az2a:h2az2a-GFP)) embryos at 48 hpf. C + D) Higher magnification, single planes of polynucleated myofibers from A + B. E) Diploid myofibers have significantly more nuclei than triploid myofibers (n = 8 embryos per ploidy, 10 fibers per embryo, p < 0.001). |
|
Vascular patterning is not significantly affected by ploidy. A + B) Composite confocal micrographs of the developing vascular system of diploid (A) and triploid (B) Tg (fli1:eGFP) embryos. ISV length and the distance between ISVs along the AP axis are not significantly affected by ploidy (n = 10 embryos per ploidy, p = 0.66 and p = 0.53, respectively). Red line indicates the plane projected in C + D (i.e. at the posterior extent of the yolk extension). C + D) Confocal projections of resliced stacks from A and B. The distance between the midline of the dorsal aorta (red line) and the lateral-most point in the ISV (green line) (i.e., mediolateral growth of the ISV) was not significantly affected by ploidy (n = 10 embryos per ploidy, p = 0.13). |
|
Triploid ISVs are not comprised of fewer cells than diploid ISVs in 48 hpf zebrafish embryos. Scale bar in panel A is valid for all panels. A + B) Composite confocal micrographs of diploid (A) and triploid (B) Tg (h2az2a:h2az2a-GFP; kdlr:mCherry) embryos, showing endothelial cell nuclei (yellow arrows). C + D) Cropped, single plane micrographs at Z-planes selected to highlight endothelial cell nuclei in the dorsal (C, 1 arrows), medial (D, 2 arrows), and ventral (D, 3 arrows) planes in a diploid embryo. E-G) Cropped, single plane micrographs at Z-planes selected to highlight endothelial cell nuclei in the dorsal (E), medial (F), and ventral (G) in a triploid embryo. There is no effect of ploidy on the number of cells in the ISVs at 48 hpf (n = 5 embryos per ploidy). |
|
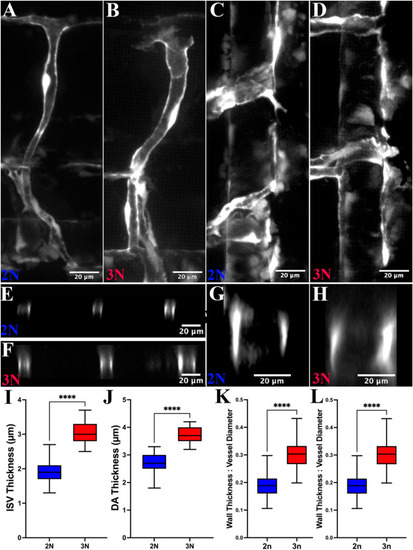
Vascular endothelial cells are thicker in triploid zebrafish. A + B) Lateral view of confocal projections of a diploid (A) and triploid (B) ISV and a section of the dorsal aorta in a diploid (C) and triploid (D) Tg (fli1:eGFP) embryo. E + F) Optical section of an ISV from A (E, diploid) and B (F, triploid). G + H) Optical section of the dorsal aortal from C (G, diploid) and D (H, triploid). I + J) Blood vessel walls of ISVs (I) and the dorsal aorta (J) are thicker (normalized to blood vessel diameter in panels (K) and (L)) in triploid than in diploid embryos (red and blue, respectively; n = 10 embryos per ploidy, p < 0.001 in both cases). |
Reprinted from Developmental Biology, 509, Small, C.D., Benfey, T.J., Crawford, B.D., Tissue-specific compensatory mechanisms maintain tissue architecture and body size independent of cell size in polyploid zebrafish, 85-96, Copyright (2024) with permission from Elsevier. Full text @ Dev. Biol.