- Title
-
High-resolution tracking of unconfined zebrafish behavior reveals stimulatory and anxiolytic effects of psilocybin
- Authors
- Braun, D., Rosenberg, A.M., Rabaniam, E., Haruvi, R., Malamud, D., Barbara, R., Aiznkot, T., Levavi-Sivan, B., Kawashima, T.
- Source
- Full text @ Mol. Psychiatry
|
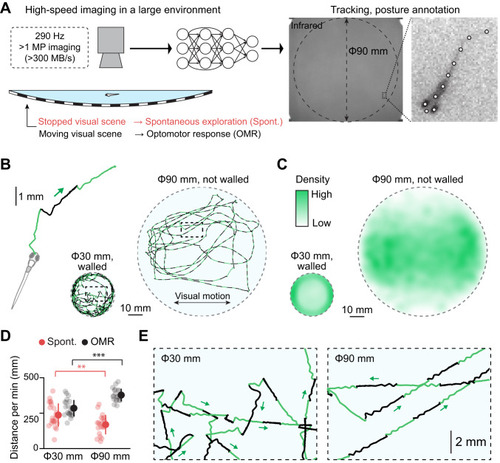
High-resolution, high-speed tracking of zebrafish behavior in a large environment. |
|
Large environment expands behavioral repertoires with less confinement artifacts. |
|
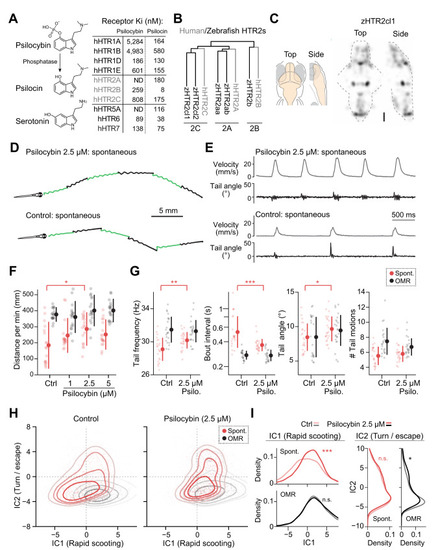
Psilocybin has stimulatory effects on spontaneous exploration. |
|
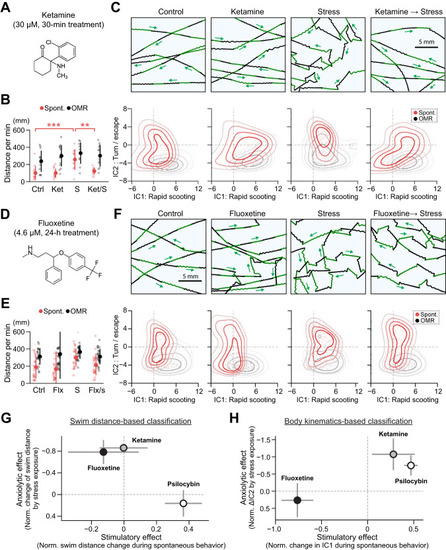
Psilocybin prevents stress-induced behavioral changes. |
|
Comparison with fast-acting and slow-acting antidepressants. |
|
Psilocybin modulates the activity of the serotonergic system. |