- Title
-
Wdr5-mediated H3K4me3 coordinately regulates cell differentiation, proliferation termination, and survival in digestive organogenesis
- Authors
- Zhang, Z., Yang, C., Wang, Z., Guo, L., Xu, Y., Gao, C., Sun, Y., Zhang, Z., Peng, J., Hu, M., Jan Lo, L., Ma, Z., Chen, J.
- Source
- Full text @ Cell Death Discov
|
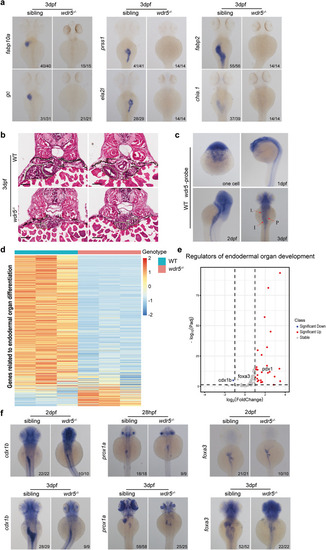
The differentiation of intestine, liver, and exocrine-pancreas is impaired in |
|
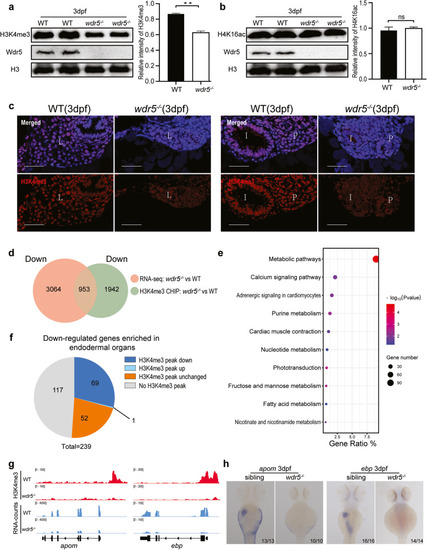
Wdr5 promotes the differentiation of digestive organs through H3K4me3, but not H4K16ac. |
|
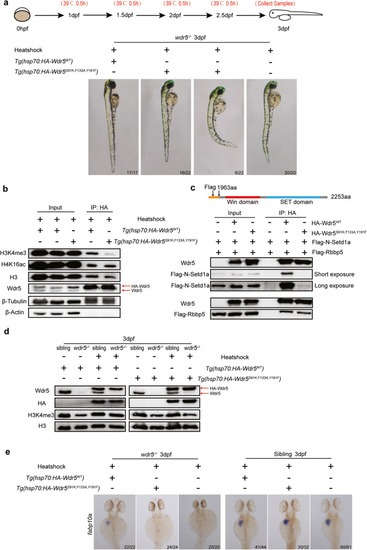
Wdr5-mediated H3K4me3 is required for digestive organ differentiation. |
|
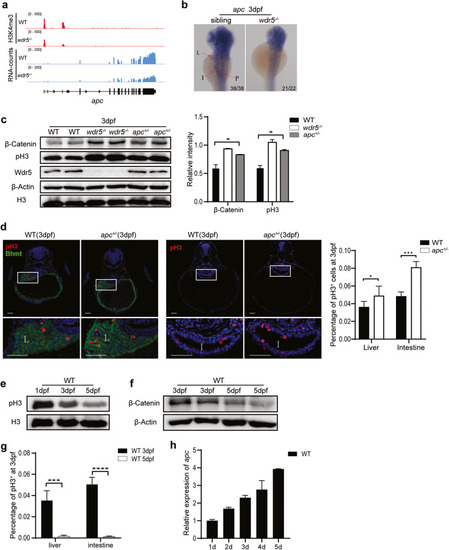
Overactivation of Wnt/β-Catenin signal is responsible for the increase of cell proliferation in |
|
Wdr5-mediated H3K4me3 upregulates |
|
The increase of apoptotic activity in |
|
Wdr5-mediated H3K4me3 promotes differentiated cell survival partially by upregulating the expression of |