- Title
-
Disruption of MAP7D1 Gene Function Increases the Risk of Doxorubicin-Induced Cardiomyopathy and Heart Failure
- Authors
- Li, L.P., Zhong, J., Li, M.H., Sun, Y.C., Niu, Y.J., Wu, C.H., Zhou, J.F., Norton, N., Li, Z.Q., Shi, Y.Y., Xu, X.L., Ding, Y.H.
- Source
- Full text @ Biomed Res. Int.
|
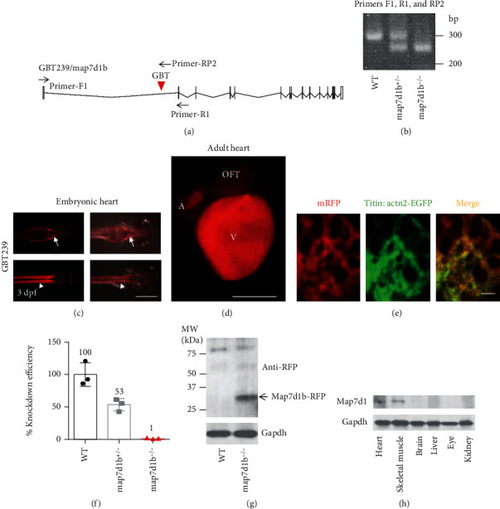
A transposon insertion in the GBT239 mutant disrupted the map7d1b gene that is predominantly expressed in the cardiac and skeletal muscle. (a) Insertional position of a gene-break transposon (GBT) element into the first intron of the map7d1b gene in the GBT239 mutant. (b) Representative DNA gel images of PCR genotyping for identifying GBT239 heterozygous (map7d1b+/-) and GBT239 homozygous (map7d1b-/-) mutant alleles. (c) Imaging the GBT239 mutant at 3 days postfertilization (dpf) reported the cardiac (arrows) and skeletal muscle (arrowheads) specific expression of the tagged Map7d1b protein. Scale bar: 0.5 mm. (d, e) Imaging the GBT239 adult heart indicated ventricle enriched expression of the tagged Map7d1b protein (d), which largely overlapped with the EGFP signal in the sarcomere reporter line Tg(titin:actn2-EGFP) (e). V: ventricle; A: atrium; OFT: outflow tract. Scale bar in (d): 1 mm. Scale bar in (e): 20 μm. (f) Quantitative RT-PCR analysis of the native map7d1b transcript disruption in the GBT239/map7d1b mutant. (g) Western blotting analysis of the Map7d1b-RFP fusion protein in the GBT239/map7d1b mutant. Arrow indicates the predicted size of Map7d1b-RFP fusion protein. (h) Western blotting analysis of the Map71b protein in different mouse tissues. EXPRESSION / LABELING:
|
|
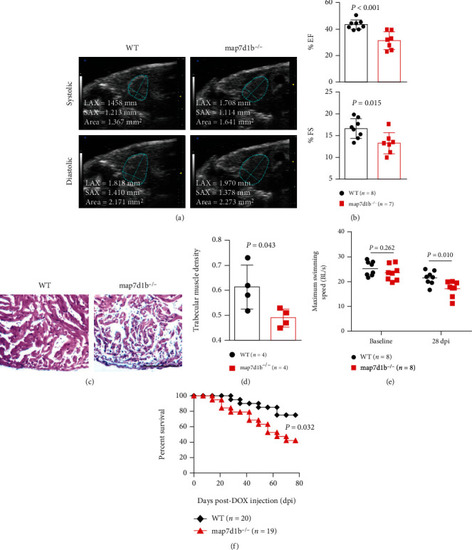
Disruption of map7d1b gene in the GBT239 homozygous mutant exacerbated doxorubicin-induced cardiac dysfunction and heart failure. (a) Shown are examples of echocardiography images extracted from movies of beating hearts in WT controls and GBT239/map7d1b homozygous (map7d1b-/-) mutants at systole (upper panel) and diastole (lower panel) contraction. (b) Quantification of cardiac function indices of ejection fraction (EF) and fractional shortening (FS) measured by echocardiography in the map7d1b-/- mutant compared to WT control at 4 weeks postdoxorubicin injection. n=7-8, Student’s t-test. (c) Representative images of H&E staining of the ventricles at 4 weeks postdoxorubicin injection. Scale bar: 100 μm. (d) Quantification of trabecular muscle density in the map7d1b-/- mutant compared to WT controls at 4 weeks postdoxorubicin injection. n=4, Student’s t-test. (e) Maximum swimming speed of the map7d1b-/- mutant compared with the WT control at both baseline and 28 days postdoxorubicin injection (dpi). n=8, Student’s -test. (f) Kaplan-Meier survival curves of WT and map7d1b-/- mutant zebrafish injected with a single bolus of 20 μg/gram body mass (gbm). The map7d1b-/- mutant had a significantly reduced survival than WT controls. n=19–20, log rank test. PHENOTYPE:
|
|
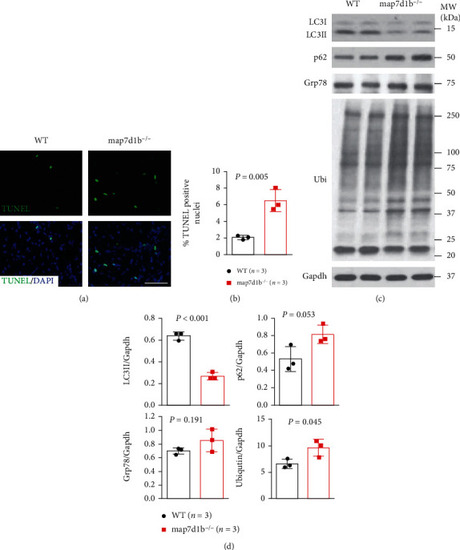
The GBT239/map7d1b homozygous mutant exhibited increased apoptotic cardiomyocyte death concurrent with impaired autophagy and elevated protein aggregation upon doxorubicin stress. (a, b) Representative images of the TUNEL assay (a) and quantification of the percentage of TUNEL-positive nuclei in GBT239/map7d1b homozygous mutant (map7d1b-/-) compared to WT controls at 4 weeks postdoxorubicin injection. n=4, Student’s t-test. Scale bar: 20 μm. (c, d) Representative Western blot images (c) and quantification analysis (d) of the expression levels of autophagy molecular markers LC3II and p62 (SQSTM1), ER stress marker glucose-regulated protein 78 (Grp78), and ubiquitin aggregated protein examined in the heart tissues isolated from the map7d1b-/- mutant compared to WT control at 4 weeks postdoxorubicin injection. n=3, Student’s t-test. PHENOTYPE:
|
|
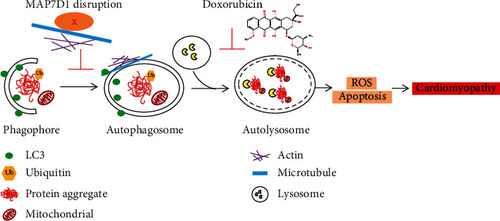
Working model of MAP7D1 in doxorubicin-induced cardiomyopathy. Doxorubicin causes cardiotoxicity by inhibiting autolysosome formation, resulting in elevated ROS and cardiomyocyte apoptosis. Disruption of MAP7D1 protein function further impaired autophagosome formation and led to accumulation of toxic protein aggregation, thus exacerbating doxorubicin-induced cardiomyopathy and heart failure. |