- Title
-
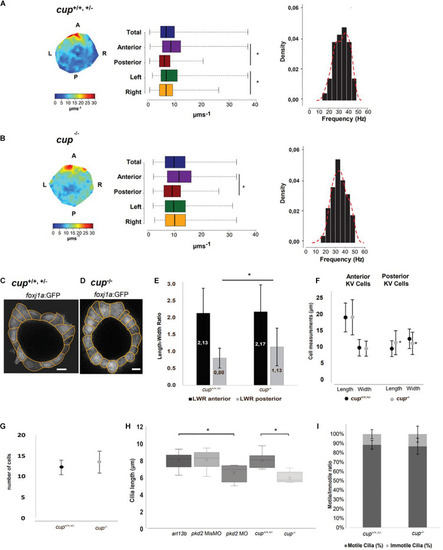
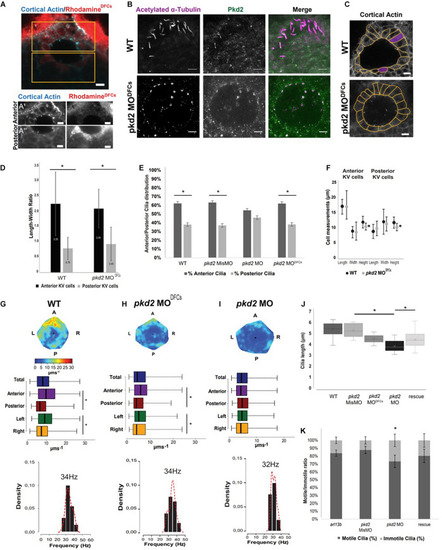
Pkd2 Affects Cilia Length and Impacts LR Flow Dynamics and Dand5
- Authors
- Jacinto, R., Sampaio, P., Roxo-Rosa, M., Pestana, S., Lopes, S.S.
- Source
- Full text @ Front Cell Dev Biol
|
|
|
|
|
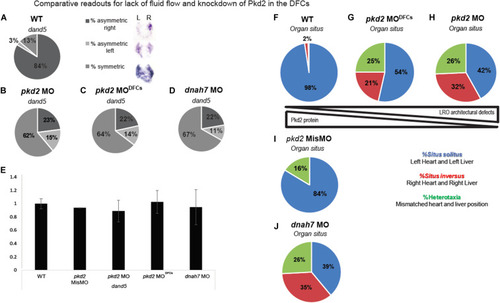
Comparative readouts for lack of fluid flow and knockdown of Pkd2 in the DFCs |