- Title
-
Loss of dmrt1 restores female fates in the absence of cyp19a1a but not rbpms2a/b
- Authors
- Romano, S., Kaufman, O.H., Marlow, F.L.
- Source
- Full text @ Development
|
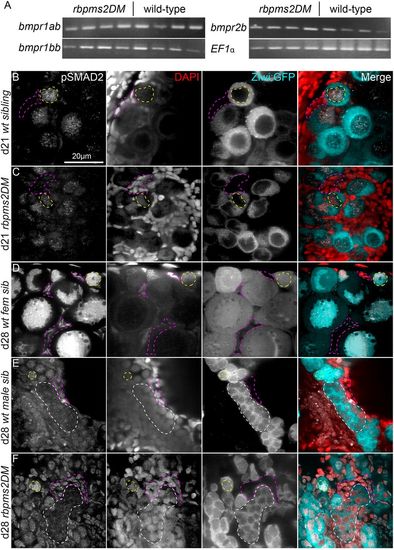
Molecularly bipotential juvenile gonads of rbpms2DMs and activation of p-SMAD in early germ cells and differentiating gonads. (A) RT-PCR on dissected trunks from individual d21 rbpms2DMs and wild-type siblings reveals no differences in expression of relevant receptors. (B-F) p-Smad2, DAPI and ziwi:GFP staining. Representative slices from Z-series reveal localization of p-Smad2 in bipotential and early differentiating gonads of d21 and d28 juvenile zebrafish, respectively. In undifferentiated bipotential ovaries of both wild-type (n=3) (B) and rbpms2DM (n=3) (C), p-Smad2 is detected in the nuclei of germ cells (example outlined with yellow dashed line) but not the nuclei of the surrounding somatic cells (examples outlined in pink dashed lines). (D) By d28 in early differentiated females (n=4), p-Smad2 remained localized to the germ cell nuclei and began to be expressed in somatic nuclei. Similarly, p-Smad2 was expressed at d28 in both germ cell nuclei (examples outlined in white dashed lines) and somatic nuclei of wild-type males (n=4) (E) and rbpms2DM gonads (n=4) (F). ‘Wild-type’ denotes wild-type or heterozygous animals as well as rbpms2a or rbpms2b single mutants that are heterozygous or homozygous for the wild-type allele at the other rbpms2 locus because these animals are phenotypically normal. |
|
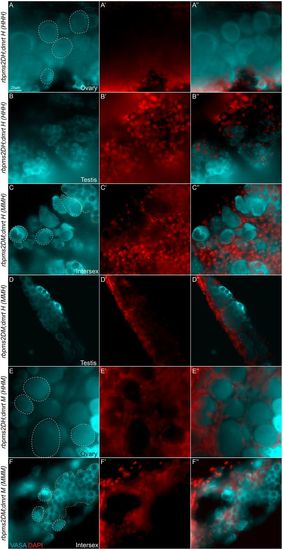
Rbpms2 is required for female gonad differentiation even in the absence of an essential male fate regulator. (A-F″) Vasa (A-F), DAPI (A′-F′) and merged (A″-F″) staining. Representative slice from a Z-stack reveals gonad development of d28 juvenile zebrafish, including female (n=1/3) (A) or male (n=2/3) (B) triple heterozygote (HHH) siblings. All rbpms2DM (MMH) gonads recovered were either intersex (n=2/3) (C) or male (n=1/3) (D). The dmrt1 mutant siblings (HHM) were female (n=3/3) (E) and triple mutant siblings (MMM) were intersex (n=3/3) (F). Oocyte-like cells are outlined in white dashed lines and testis-like cells in orange dashed line. |
|
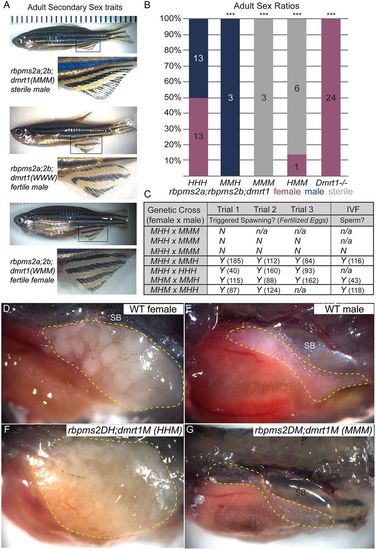
rbpms2;dmrt1TMs are sterile, unlike rbpms2DMs. (A) Secondary sex traits were assessed to identify fish from each category. Representative images show body shape and color; insets are magnified views of the anal fin. (B) Adult sex ratios of progeny pooled from three triple heterozygote intercrosses for given genotypes. All double mutants also heterozygote for dmrt (MMH) were male as previously observed in rbpms2DMs, and all dmrt1 mutants in any other genetic combination (HHM, MHM, etc.) were female, as previously reported for dmrt1uc27/uc27. All triple mutants (MMM) were sterile males. Numbers indicate number of animals for each condition. Animals mutant for dmrt1 and any combination of a wild-type or heterozygous for a rbpms2 allele (WMM, WHM, HHM, etc.) were combined because they were phenotypically wild type, with the exception of rbpms2;dmrt1HMM as it had a different phenotype. The X2 goodness of fit test with Bonferroni correction was used for multiple comparisons. ***P<0.001 indicates groups that deviated from the expected 50:50 sex ratio. (C) Fertility was assessed in mating trials as indicated in the chart. For each cross, if a male triggered spawning or if sperm was acquired through IVF, the numbers (in parentheses) of fertilized eggs per trial for natural breeding are indicated. n/a indicates fish that were not able to be mated for subsequent mating trials. (D-G) Representative lateral view images of representative adult with tissue resected to reveal gonads (outlined in yellow dashed lines) of wild-type female (D), wild-type male (E), dmrt1uc27/uc27 (rbpms2;dmrt1HHM) female (F) and rbpms2;dmrt1MMM (G) fish. TM fish lacked morphologically obvious gonads proximal to the swim bladder (SB) compared with fertile sibling wild type or single mutants. |
|
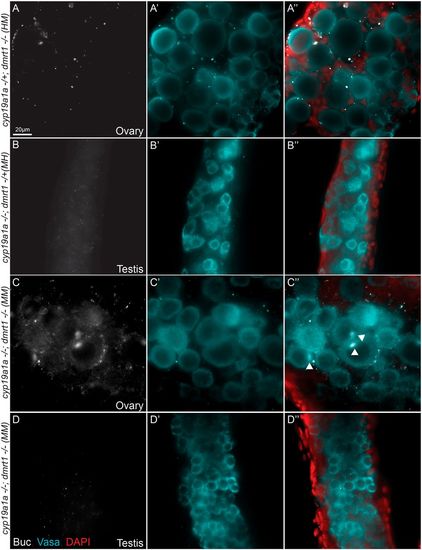
Cyp19a1a promotes bipotential and female development by inhibition of dmrt1. (A-D″) Buc (A-D), Vasa (A′-D′) and Buc/Vasa/DAPI merged (A″-D″) staining in representative slices from individual Z-series allows visualization of gonocyte development and sex-specific differentiation in d45 juvenile zebrafish, including female cyp19a1auc38/+;dmrt1uc27/uc27 (HM) (n=3/3) (A), male cyp19a1auc38/uc38;dmrt1uc27/+ (MH) (n=5/5) (B) and cyp19a1auc38/uc38;dmrt1uc27/uc27 (MM) gonads that were either male (n=1/4) with male germ cells (C) or female (n=3/4) with oocytes (D), as shown by expression of the oocyte marker Buc (white) and indicated with white arrowheads. |
|
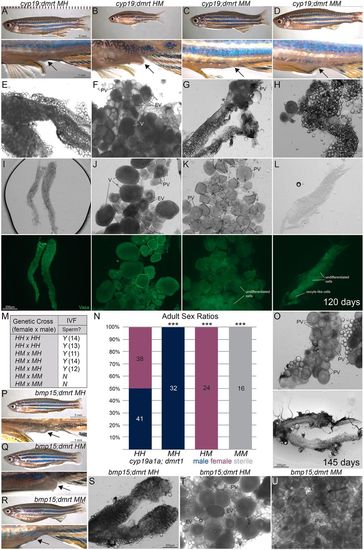
Cyp19a1a is required for oocyte maturation and maintenance of female fates, independently of antagonism of dmrt1. (A-D) Representative lateral view images show the external secondary sexual characteristics of d120 adults: cyp19a1a;dmrt1MH (n=7) male (A), cyp19a1a;dmrt1HM (n=7) female and cyp19a1a;dmrt1MM (n=5) mutants (C,D). Male MH fish (A) had a slender body shape and lacked a genital papilla (indicated by arrow). Female HM fish (B) had a round body shape with protruding abdomen and extended genital papilla. Similar to MH males, MMs (C,D) exhibited male secondary sex traits. (E-H) However, upon dissection, MH males (E) had testes, HM females (F) had ovaries that contained pre-vitellogenic (PV) early-vitellogenic (EV) and vitellogenic (V) stages of oocytes, whereas MMs (G,H) had gonads that were atypical in overall structure and contained oocytes that only progressed to the PV stage. (I-L) Additional representative brightfield and Vasa (green) overview images of cyp19a1a;dmrt1MH (I), cyp19a1a;dmrt1HM (J) and cyp19a1a;dmrt1MM (K,L) gonads. (M) Fertility was assessed by natural mating or by IVF extraction of sperm followed by fertilization of eggs. For each cross or if sperm was acquired through IVF, the numbers (in parentheses) of fertilized eggs per trial are indicated. (N) Adult sex ratios of progeny pooled from heterozygote intercross and double heterozygote crossed to a mutant;heterozygote (HH×MH) for given genotypes. All cyp19a1auc38 mutants that were also heterozygous for dmrt1 (MH) were males, all dmrt1uc27 mutants that were also heterozygous for cyp19a1auc38 (HM) were female. All cyp19a1auc38/uc38;dmrt1uc27/uc27 double mutants appeared male based on secondary sex traits, but female based on primary gonad sex and were classified as sterile because of the lack of mature gametes. Numbers indicate individual animals for each condition. X2 goodness of fit test with Bonferroni correction was used for multiple comparisons. ***P<0.001 indicates groups that deviated from the expected 50:50 sex ratio. (O) Representative gonads from two d145 cyp19a1a;dmrt1MM fish (total, n=4) show an ovary containing PV oocytes and a gonad lacking oocytes. (P-R) Representative lateral view images show the external secondary sex traits of d145 bmp15;dmrt1 adults. The bmp15;dmrt1MH (n=7) (P) and bmp15;dmrt1HM (n=7) (Q) fish display normal secondary sex characteristics of males and females, respectively. MMs (n=4) (R) had a female body shape but lacked protruding genital papillae. (S-U) Upon dissection, MH males (S) had testes, HM females (T) had ovaries and MMs (U) had ovaries that contained PV oocytes. |
|
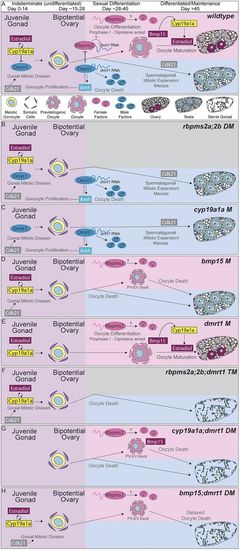
Model: Cyp19a1a inhibits dmrt1 to promote bipotential ovary formation and acquisition of female fates, which requires rbpms2 even in the absence of dmrt1. (A-F) Timeline (from left to right) of requirement for each essential regulator of sex determination. (A) Cyp19a1a is required for the transition from juvenile gonad to bipotential ovary by antagonizing Dmrt1 activity. Cdk21 promotes mitotic divisions of gonocytes, whereas Amh limits gonial proliferation within the bipotential ovary. Inhibition of Dmrt1 allows expression of Rbpms2, either directly or indirectly, and female development in the absence of Cyp19a1a. Rbpms2 probably promotes female fates temporally upstream of Bmp15 and estrogen (estradiol), which promote follicle maturation beyond pre-vitellogenic phases. Cyp19a1a is an enzyme that functions to catalyze the final step of estrogen synthesis; therefore, the antagonistic relationship between Cyp19a1a and Dmrt1 is probably an indirect relationship mediated by the estrogens produced by Cyp19a1a. Although Cyp19a1a is dispensable to establishment of the bipotential ovary and initiation of female development in the absence of Dmrt1, it appears to be required for subsequent oocyte maturation and maintenance of female fates independently of antagonism of Dmrt1. Bmp15 produced in oocytes is required to promote oocyte differentiation and cyp19a1a-positive granulosa cells, indicating that the subsequent failure of cyp19a1a;dmrt1 double mutant oocytes to mature is a result of loss of the later granulosa cell-derived source of Cyp19a1a, which appears not to require inhibition of Dmrt1. (B-H) Model of mutant scenarios for each essential regulator. (B) In rbpms2a;rbpms2b double mutants, Rbpms2 repression of dmrt1 transcript is alleviated and Dmrt1 is translated, leading to male differentiation. Within the testis, Cdk21 promotes spermatogonial expansion and meiosis. (C) In cyp19a1a mutants, Cyp19a1a no longer antagonizes dmrt1, probably due to reduced estradiol synthesis, and Dmrt1 is expressed prematurely, leading to precocious male differentiation. (D) In bmp15 mutants, oocytes do not progress past the PV stage, in part because Bmp15 is required for late Cyp19a1a production. (E) In dmrt1 mutants, Dmrt1 is not present to antagonize Rbpms2, and female differentiation ensues. (F) In rbpms2a;rbpms2b;dmrt1 triple mutants, female differentiation cannot occur in the absence of Rbpms2, whereas male differentiation cannot proceed because of loss of Dmrt1, resulting in the formation of a sterile testis. (G) In cyp19a1a;dmrt1 double mutants, loss of dmrt1 is sufficient to restore the transition from juvenile gonad to bipotential ovary and to prevent precocious male differentiation as occurs in cyp19a1a mutants. However, despite the absence of dmrt1, oocytes lacking all Cyp19a1a (cyp19a1a;dmrt1 double mutants) or (H) Bmp15-dependent Cyp19a1a (bmp15;dmrt1 double mutants) fail to progress to vitellogenic stages and female development ultimately fails, indicating that Cyp19a1a promotes oocyte progression independently of dmrt1 antagonism. Probably because of later requirements for dmrt1 in testis development, loss of both dmrt1 and cyp19a1a or bmp15 eventually results in sterile male adults. |