- Title
-
Visualization of Mitochondrial Ca2+ Signals in Skeletal Muscle of Zebrafish Embryos with Bioluminescent Indicators
- Authors
- Vicente, M., Salgado-Almario, J., Soriano, J., Burgos, M., Domingo, B., Llopis, J.
- Source
- Full text @ Int. J. Mol. Sci.
|
Expression of biosensors GFP-aequorin (GA) and mitoGA in zebrafish embryos. ( |
|
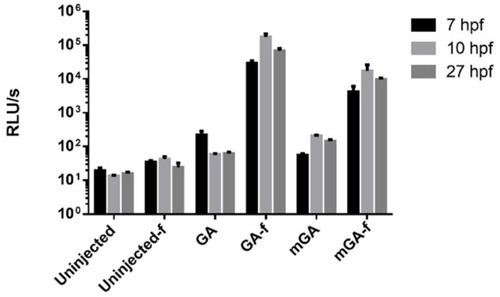
Aequorin reconstitution of GA and mitoGA (mGA) with |
|
Continuous luminescence imaging of spontaneous Ca2+ transients in the trunk of zebrafish from 12 to 28 hpf. ( |
|
Frequency of spontaneous contractions and Ca2+ transients in the trunk of zebrafish embryos (24–28 hpf). ( |
|
Effect of inhibiting mitochondrial Ca2+ uptake on the frequency of contractions and Ca2+ transients in 24–28 hpf embryos. Effect of the uncoupler FCCP (1 µM) and the MCU inhibitor DS16570511 (100 µM) on the frequency of skeletal muscle contractions in the trunk in non-injected embryos ( |
|
Kinetics of spontaneous Ca2+ transients in the trunk and tail in 28 hpf zebrafish embryos. ( |
|
Comparison of the kinetic parameters of cytosolic and mitochondrial Ca2+ transients in zebrafish trunk in embryos expressing GA and mitoGA. Ca2+ transients in cytoplasm and mitochondria were compared. Embryos were imaged at 11.9 Hz. A two-tailed unpaired |
|
Propagation of Ca2+ transients along the trunk and tail in embryos expressing Twitch-4 and GA. ( |