- Title
-
Transgenic zebrafish illuminate the dynamics of thyroid morphogenesis and its relationship to cardiovascular development
- Authors
- Opitz, R., Maquet, E., Huisken, J., Antonica, F., Trubiroha, A., Pottier, G., Janssens, V., and Costagliola, S.
- Source
- Full text @ Dev. Biol.
|
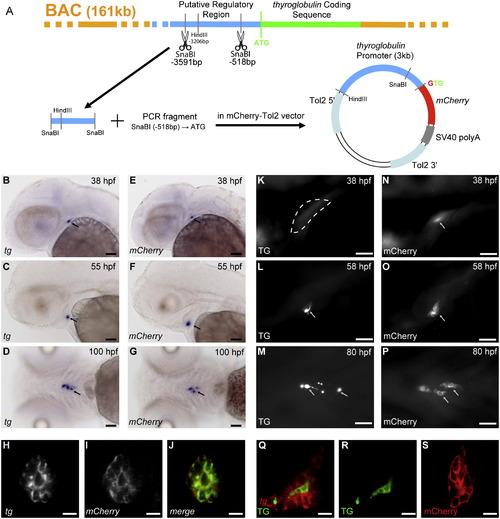
Generation and characterization of tg(tg:mCherry) transgenic embryos. (A) Cloning strategy: A 3 kb region of the putative regulatory region of the zebrafish thyroglobulin coding sequence was isolated from a BAC clone by restriction with SnaBI. PCR was used to generate the remaining sequence upstream of the ATG, and a 3.2 kb DNA fragment was finally cloned upstream of the mCherry coding sequence. The final construct, flanked by Tol2 sites, was injected at the one-cell stage embryo. ((B)-(G)) mCherry mRNA expression in transgenic embryos recapitulates the thyroid-specific expression of endogenous tg mRNA as shown by whole-mount in situ hybridization. Arrows point to the thyroid. ((H)-(J)) Double fluorescent in situ hybridization (FISH) confirmed coexpression of mCherry and tg mRNA in all thyroid cells. Confocal images of a 55 hpf embryo are shown. ((K)-(P)) Whole-mount immunofluorescence (IF) of transgenic embryos revealed mCherry protein expression ((N)-(P)) as early as 36/37 hpf, well before thyroglobulin (TG) protein ((K)–(M)) becomes detectable around 55 hpf. The encircled area in panel (K) marks the thyroid region which lacks staining for TG protein at 38 hpf. Arrows point to the thyroid. ((Q)-(S)) TG staining is limited to the colloid (see (Q) and (R)) whereas mCherry IF staining labels all thyroid cells (see (S)). Panels (Q) and (R) show a confocal section of the thyroid of a 60 hpf embryo after combined staining for tg mRNA (FISH) and TG protein (IF). Panel (S) shows a confocal section of the thyroid of a 55 hpf embryo after IF staining for mCherry. Ventral views are shown in (D), (G), (M) and (P), all other images show lateral views. All embryos shown are oriented with anterior to the left. Scale bar: 50 μM in (B)-(G), (K)-(P) and 10 μM in (H)-(I), (Q)-(S). |
|
Early onset of reporter expression allows to monitor budding and early relocation of thyroid in live tg(tg:mCherry) embryos. (A) Lateral view of a live tg(tg:mCherry) embryo at 45 hpf showing strong red fluorescence in the thyroid primordium (arrow) during thyroid budding. ((B)–(D)) Stills from a time-lapse movie showing thyroid budding and detachment from the pharyngeal floor in a tg(tg:mCherry;sox17:EGFP) embryo. EGFP labels the pharyngeal endoderm. Live imaging was started at 44 hpf and lasted for 10 h. The timer indicates the time elapsed since the beginning of live imaging. Lateral views are shown, anterior is to the left. The asterix denotes the position of pharyngeal pouch 1. The dotted line facilitates visualization of relative thyroid bud (arrow in (B)) movement. ((E)-(G)) The thyroid primordium maintains epithelial characteristics during budding and relocation into the subpharyngeal mesenchyme as demonstrated by E-cadherin expression. Confocal images of sagittal vibratome sections are shown for tg(tg:mCherry) embryos after IF staining for mCherry and E-cadherin. Anterior is to the left and dorsal is up. Scale bar: 50 μM in B-D; 20 μM in (E)-(G). |
|
Early thyroid morphogenesis occurs in close spatial proximity to the apical pole of the heart. ((A)-(D)) Stills from a time-lapse movie of a tg(tg:mCherry;myl7:EGFP) embryo showing that thyroid budding and relocation from the pharynx is highly coordinated with the descent of the heart. EGFP labels the myocardium and mCherry the thyroid. Live imaging was started at 38 hpf and lasted for 17 h. The timer indicates the time elapsed since the beginning of live imaging. Lateral views are shown. The dotted line facilitates visualization of the relative movement of the thyroid (arrow in (A)). ((E)-(J)) Whole-mount IF staining of tg(tg:mCherry;myl7:EGFP) embryos confirmed the intimate contact between thyroid primordium and distal ventricular myocardium during thyroid invagination from the pharynx. Panels (E)-(G) show epifluorescence images of whole mount embryos. Panels (H)-(J) show confocal images of sagittal vibratome sections of stained embryos. mCherry, EGFP and E-Cadherin label thyroid primordium, myocardium and foregut epithelium, respectively. In all panels, anterior is to the left. Scale bar: 50 μM. |
|
Rostral expansion of thyroid tissue along the pharyngeal midline occurs coordinatedly with pharyngeal vessel remodeling. (A) Ventral view of a live tg(tg:mCherry) embryo at 70 hpf displaying strong red fluorescence of the rostrally expanding thyroid tissue (arrow). ((B)-(F)) Stills from a time-lapse movie of a tg(tg:mCherry;fli1a:EGFP) embryo showing that thyroid expansion along the pharyngeal midline occurs concomitantly with a general remodeling of the hypobranchial region. EGFP labels endothelial cells and neural crest-derived mesenchyme. Live imaging was started at 75 hpf and lasted for 26 h. The timer indicates the time elapsed since the beginning of live imaging. Ventral views are shown, anterior is to the top. ((G)-(L)) Whole-mount IF staining of GFP and mCherry for a series of fixed tg(tg:mCherry;kdrl:EGFP) embryos shows developmental changes in gross thyroid morphology relative to the remodeling pharyngeal vasculatures. EGFP labels endocardium and vascular endothelium. Ventral views are shown and anterior is to the top. ((M) and (N)) Live imaging of tg(tg:mCherry;kdrl:EGFP) embryos by selective plane illumination microscopy (SPIM) identified the hypobranchial artery (HA) as the main vessel contacting the thyroid during the initial stages of its rostral expansion. (M) shows a single frame from time-lapse Movie 6. (N) shows a magnified view of the outflow tract (OFT) region. M and N show ventral views, anterior is to the left. ((O) and (P)) The close contact between thyroid cells and endothelial cells of the forming HA was confirmed by confocal microscopy of tg(tg:mCherry;kdrl:EGFP) embryos after IF staining for mCherry and GFP. Confocal sections of sagittal vibratome sections are shown, anterior is to the left. ((Q)–(W)) Confocal microscopy of a developmental series of tg(tg:mCherry;kdrl:EGFP) embryos after IF staining for mCherry and GFP demonstrate the location of thyroid tissue relative to the HA and VA. During the initial rostral expansion of the thyroid (55–70 hpf), the thyroid was in close contact with the HA while the VA, along its entire length, was still located caudal to the heart OFT. Thereafter, a progressive rostral protrusion of the VA relative to the OFT was evident (see (R)–(T)). Between 70 and 75 hpf, the anterior end of the VA (aVA) was located at the level of the thyroid, while the major part of the VA was still located caudal to the OFT (see (R)). Around 100 hpf, the entire VA was located anterior to the OFT (see (T)). By that time, the rostral limit of the VA was located anteriorly to the thyroid. Note that the location of the most anterior thyroid tissue was invariably determined by the location of the HA branching point (see (O),(P),(U) and (V)). The caudally running paired HA demarcates the lateral extension of the dispersed thyroid follicles (see (W)). Panels (Q)-(U) show confocal images acquired from sagittal vibratome sections. Panels (V) and (W) show confocal images acquired from frontal vibratome sections. aa1, aa3, aa5, aortic arch arteries 1, 3, 5; as, aortic sac; (H), heart; HA, hypobranchial artery; ORA, opercular artery; VA, ventral aorta. Scale bars: 50 μM in panels (G)-(L), (N), (Q)–(W), 100 µM in panel M, 20 μM in panels (O) and (P). |
|
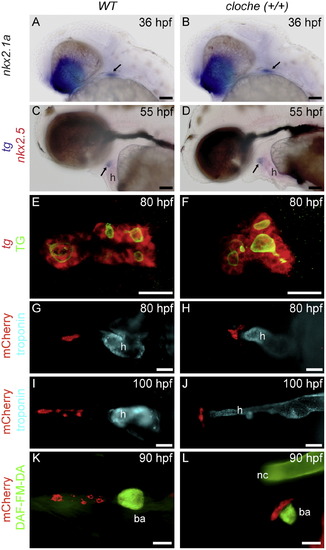
Absence of pharyngeal vessels in cloche mutant embryos affects late thyroid relocation. ((A)-(D)) Whole-mount in situ hybridization for thyroid markers (nkx2.1a, tg) shows that early thyroid morphogenesis is not grossly affected in cloche mutant embryos. Arrows point to the thyroid. ((E)-(F)) Follicle formation appears grossly normal in cloche mutant embryos as demonstrated by double staining for tg mRNA (using FISH) and TG protein (using IF). Images in (E) and (F)show confocal projections. ((G)-(L)) Cloche embryos expressing mCherry in the thyroid were analyzed for possible late thyroid phenotypes. Whole-mount IF for mCherry and cardiac troponin T (labelling cardiac myocardium) revealed clear defects in thyroid relocation (see (G)-(J)). While wild-type embryos showed rostral expansion of thyroid tissue along the pharyngeal midline, thyroid tissue remained clustered around the outflow tract in cloche mutant embryos as shown by DAF-FM-DA labelling of the bulbus arteriosus (see panels (K) and (L)). Lateral views are shown in (A)-(D) and (L), ventral views are shown in (E)-(K), and anterior is to the left in all images. h, heart; ba, bulbus arteriosus; nc notochord. Scale bar: 50 μM in (A)-(D), (G)-(L); 20 μM in (E) and (F). |
|
ltbp3 morphant embryos develop distinct vascular and thyroid defects. tg(tg:mCherry;kdrl:EGFP) embryos injected with a control morpholino (CTRL-MO) or a morpholino targetting ltbp3 (ltbp3-MO) were stained by IF for mCherry (red), GFP (green) and cardiac troponin T (white) and were analyzed by confocal microscopy for thyroid and vessel defects at 55, 80 and 100 hpf. ((A)-(C)). Embryos injected with a CTRL-MO displayed normal development of the pharyngeal vasculature and thyroid tissue. Note that the compact mass of thyroid cells at 55 hpf was tightly invested by endothelial cells of the forming hypobranchial artery (HA). Later, control embryos displayed the characteristic expansion of thyroid tissue along the pharyngeal midline closely apposed to the ventral aorta (VA) and the caudally running paired HA. ((D)-(F)) The majority of ltbp3 morphants (group I) displayed a thyroid phenotype that was characterized by a lateral expansion of thyroid tissue in the vicinity of the heart outflow tract (OFT). Note that lateral expansion was evident as early as 55 hpf (D) and was associated with the presence of dysplastic HA. The HA was absent at later stages and a VA was never formed ((E) and (F)). ((G)-(I)) The other distinct thyroid phenotype observed in ltbp3 morphants (group II) was characterized by a single compact mass of thyroid cells in the vicinity of the OFT. In ltbp3 morphants of group II, formation of the HA was never observed and the thyroid showed neither a rostral expansion along the midline nor a lateral expansion. All panels show ventral views and anterior is to the left. aa1, aa3, aortic arch artery 1 and 3; ba, bulbus arteriosus. Scale bar: 50 μM. |
|
Treatment of embryos with the VEGFR tyrosine kinase inhibitor vatanalib from 20 to 55 hpf caused severe defects in the pharyngeal vasculature. tg(tg:mCherry;kdrl:EGFP) embryos treated with vatanalib (5 µM) or DMSO (0.1%) were stained by IF for mCherry (red) and GFP (green) and were analyzed by confocal microscopy for thyroid and vessel defects at 80 hpf. Note that some embryos were also stained for cardiac troponin T (white). (A) Analysis of DMSO-treated embryos at 80 hpf showed a normal vascular development with the thyroid tissue showing a progressive expansion along the pharyngeal midline. ((B)–(F)) Vatanalib treatment resulted in a variety of defects in ventral aorta (VA), hypobranchial artery (HA) and aortic arch artery (aa1, aa3) formation. While a highly dysplastic HA could be identified in many embryos (see (B) and (C)), in some embryos it was not possible to unequivocally identify the vessels present in the highly irregular networks close to the outflow tract (see (D)). The thyroid tissue of vatanalib-treated embryos was always located in close vicinity to vessels so that any deviation of thyroid tissue from the midline was associated with abnormal vessel localization. ((E)-(F)) Notably, whenever the HA could be identified, thyroid tissue was found associated with it. Scale bar: 50 μM). |
|
Detection of ectopic thyroid cells by epifluorescence microscopy in live (tg:mCherry;sox17:EGFP) embryos. Panels A and B show lateral views of a 60 hpf embryo presenting a small group of ectopic fluorescent thyroid cells (e) located within the EGFP-positive pharyngeal epithelium. The main mass of thyroid cells (arrow) showed normal relocation from the pharynx into the subpharyngeal mesenchyme. Panels C and D show results from whole mount in situ hybridization performed with embryos in which ectopic fluorescence was detectable or absent during live imaging. Note that tg mRNA expression could be readily detected within the pharyngeal epithelium of those embryos that displayed an ectopic reporter signal during live imaging (see panel C). In contrast, no ectopic tg mRNA expression was detected in embryos that did not show ectopic ectopic fluorescence during live imaging (see panel D). Scale bar: 50 μM. |
|
Live imaging of thyroid development in wild-type (WT) and cloche embryos expressing mCherry in thyroid cells. The two columns on the left show lateral views and the two columns on the right show ventral views of embryos that were transiently embedded in low melting agarose for live imaging at the indicated time points. The normal expansion of thyroid tissue along the pharyngeal midline seen in WT embryos was defective in cloche embryos. Note that the progressive increase of the pericardial edema caused a marked disruption of the normal morphogenesis of the hypobranchial region as a whole. Merged images from brightfield and epifluorescence microscopy are shown. Scale bar: 100 μM. |
|
Whole-mount in situ hybridization for expression of the thyroid marker nkx2.1a did not show gross effects on early thyroid morphogenesis in ltbp3 morphants. Size and location of the early thyroid primordium was not different from control embryos at 30 hpf. In addition, injection of ltbp3 morpholino (ltbp3 MO) did not affect thyroid budding from the pharynx. Arrows point to the thyroid. Scale bar: 100 μM. |
|
Panels A and B show that teatment of embryos with the VEGFR tyrosine kinase inhibitor vatanalib from 20 to 55 hpf caused severe defects in intersegmental vessel (ISV, white arrows) morphogenesis. tg(tg:mCherry;kdrl:EGFP) embryos treated with vatanalib (5 μM) or DMSO (0.1%) were fixed at 55 hpf and analyzed by IF for GFP expression in the trunk/tail region. DMSO-treated embryos showed regular spacing of ISV along the trunk. In contrast, the number of ISV detectable in vatanalib-treated embryos was dramatically reduced and the remaining ISV showed an abnormal morphology. Panels C–F show results from whole-mount in situ hybridization for expression of the thyroid marker nkx2.1a in DMSO- and vatanalib-treated embryos. Examination of vatanalib-treated embryos did not reveal gross effects on size and location of the early thyroid primordium at 30 hpf. In addition, vatanalib treatment did not affect thyroid budding from the pharynx. Black arrows point to the thyroid. Scale bar: 100 μM. |
Reprinted from Developmental Biology, 372(2), Opitz, R., Maquet, E., Huisken, J., Antonica, F., Trubiroha, A., Pottier, G., Janssens, V., and Costagliola, S., Transgenic zebrafish illuminate the dynamics of thyroid morphogenesis and its relationship to cardiovascular development, 203-216, Copyright (2012) with permission from Elsevier. Full text @ Dev. Biol.