- Title
-
Effect of Vascular Cadherin Knockdown on Zebrafish Vasculature during Development
- Authors
- Mitchell, I.C., Brown, T.S., Terada, L.S., Amatruda, J.F., and Nwariaku, F.E.
- Source
- Full text @ PLoS One
|
VE-Cad MO prevents VE-cadherin expression and impairs cardiac formation. Western blot analysis was performed on protein lysates collected from 24-hour old uninjected (Blot, left), VE-Cad MO injected (Blot, center) and control MO injected (Blot, right) embryos were probed with antibody directed against human VE-cad (110 kDa). Mouse β-actin antibody is shown as a loading control (42 kDa). Brightfield microscopy was used to obtain images of 48–96 hpf embryos injected with control MO (left column) and VE-cad translation-blocking MO (right column). At 48 hpf (a–d), low power images show similar overall body morphology between control (a) and VE-cad MO injected (b) embryos (40x). High power views of the thoracic region (d) show pericardial edema, atrial dilation and blood pooling at the sinus venosus 200x). Tracings of the atrial (blue) and ventricular (red) size and orientation are also shown. By 72 hpf (e–h), pericardial edema is prominent in knockdown embryos and the cardiac chambers retain a linear orientation (f,h) in contrast to control embryos (e,g). Control embryos appear normal at 96 hours (i,k) with normal overlap of the 2 chambers, however VE-cad MO embryos show significant pericardial edema, improper cardiac looping, and circulatory arrest (j,l). |
|
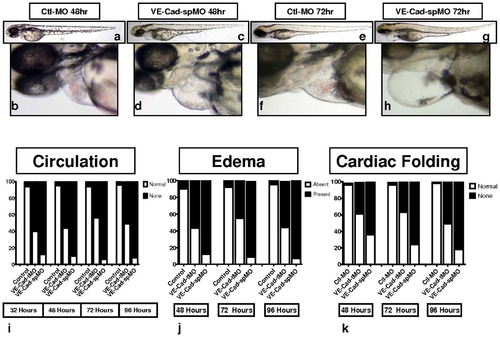
VE-Cad MO splice-blockade also impairs formation of circulation and results in pericardial edema and improper cardiac looping. Brightfield images of embryos injected with VE-Cad splice-blocking morpholino (VE-Cad spMO) also show impaired circulation, pericardial edema and impaired cardiac looping (c–d, g–h), when compared to controls (a–b, e–f). Assessments of cardiovascular phenotype: (i) circulation was assessed in VE-cad tMO, spMO and control MO embryos, with VE-cad MO injected embryos demonstrating impaired circulation at all time points. (j) Pericardial edema was highly prevalent in VE-cad MO injected embryos. (k) Cardiac looping in the two groups, with abnormal looping more frequent in the VE-cad MO fish (n = 150–200 embryos per group). |
|
Loss of VE-cadherin does not affect primitive hematopoiesis, vascular sprouting or peripheral vessel integrity. Embryos at 48 hpf were stained with O-dianisidine for erythrocytes. No overall difference is observed between control (a) and Ve-cadherin knockdown embryos (b, 40x). Transgenic fli1:gfp embryos were injected with control MO and VE-cad MO and observed by epifluorescence microscopy. Vasculogenesis and intersomitic sprouting appears unaffected by VE-Cadherin knockdown (d) at 48 hpf compared to controls. At 48 and 72 hpf, despite normal vasculature, the endocardium of knockdown embryos (d,f) shows a linear atrial (arrow) and ventricular (arrowhead) orientation within a dilated pericardium (e). Low power images (g,j, 40x) of Control-MO and VE-Cad MO injected embryos show normal sprouting of the intersegmental vessels and DLAV (arrowheads) as well as the subintestinal vessels (long arrow) in both embryos. Impaired cardiac loopin,g is noted in VE-cad MO injected embryos (j, short arrow). Medium power images of the boxed regions (h,k 200x) demonstrate similar sprouting patterns between control and knockdown embryos in the head vasculature. Images of intersegmental/DLAV junctions (i,l 300x) show intact vessels and no extravasation in either control MO or VE-cad MO injected embryos (junctions at arrowheads I,l). PHENOTYPE:
|
|
Knockdown of VE-cadherin causes defective cardiac looping and chamber thinning. In situ hybridization of Control MO and VE-cad MO injected embryos with the pan-cardiac myosin marker cmlc2 (a–h) and the ventricular-specific vmhc (i–j). Ventral and lateral images of control embryos stained for cmlc2 (a,c) show proper atrial (white arrow) and ventricular (black arrows) looping. Images of VE-Cad knockdown embryos show a linear arrangement of the cardiac chambers (b,d). By 96 hours, cmlc2 staining shows the normal side-by-side arrangement of chambers in control embryos (e,g), while the heart remains thinned and tubular in VE-cad MO injected embryos (f,h). Staining for vmhc at 72 hours shows a compact ventricular chamber in controls (i), while the ventricles of knockdown embryos are elongated and thinner-appearing (j). EXPRESSION / LABELING:
PHENOTYPE:
|
|
VE-cadherin knockdown produces persistent, excessive endocardial/myocardial separation. H+E staining at medium (300x) and high power (600x) aortic images of control (a,c) and VE-Cad knockdown (b,d) embryos at 96 hpf. The aorta of both control MO and VE-cad MO embryos appears contiguous. Medium (200x) and high power (400x) images of the thoracic region of control MO (e,g) and VE-cad MO (f,h) embryos. The atria of control embryos are intact (long arrows e,g), while those of knockdown embryos are elongated, with persistent endocardial detachment from the myocardial layer (long arrows f,h). The ventricles are intact in both control and knockdown embryos, though VE-cad MO embryos have thinner ventricular walls (short arrows e–h). Pericardial edema is present only in VE-cad MO embryos, however both types show a normal-appearing bulbus arteriosus, normal A–V valve formation (arrowheads) and blood within the aortic arches. Ao, Aorta; BA, Bulbus Arteriosus; GT, Gut Tube; PC, pericardium;Y, Yolk, Scale bars 75 μm. PHENOTYPE:
|
|
VE-cadherin knockdown in fli1:GFP embryos reproduces and allows measurement of endocardial/myocardial separation at 32 hpf. Images taken from movies of transgenic fli:GFP embryos that were injected with either control MO or VE-cad MO and observed with fluorescence microscopy (a,b). These images demonstrate increased endocardial/myocardial separation. The average measured distance between endocardium and myocardium (white arrows) differed significantly between the two groups, with the knockdown embryos consistently having a larger separation between the two layers (c). PHENOTYPE:
|
|
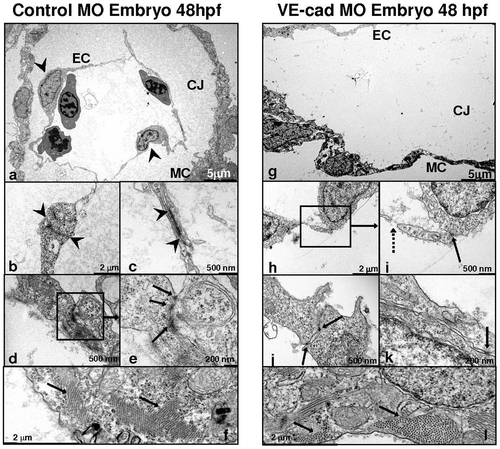
Loss of VE-cadherin produces defective endocardial junctions and increased permeability: Images obtained by transmission electron microscopy of control (a–f) and VE-cad knockdown (g–l) embryos at 48 hpf. The atria of control embryos (a) demonstrate a modest layer of cardic jelly (CJ) between the endocardial (EC) and myocardial layers (MC) (arrowheads are endothelial cell nuclei in a). Knockdown embryos (g) show a stretched myocardium, wide endocardial/myocardial separation and decreased electron density of the cardiac jelly. Mature-appearing, long endocardial junctions are present in controls (between arrowheads, b and c; arrows e). VE-cad MO embryos have smaller, fewer, less well-developed junctions (solid arrows i–k) and endothelial gaps (dashed arrow, i). Both control and knockdown embryos (f and l) demonstrate similar overall numbers of well-developed contractile elements in their myocardial layers (f and l). PHENOTYPE:
|