- Title
-
Six1a is required for the onset of fast muscle differentiation in zebrafish
- Authors
- Bessarab, D.A., Chong, S.W., Srinivas, B.P., and Korzh, V.
- Source
- Full text @ Dev. Biol.
|
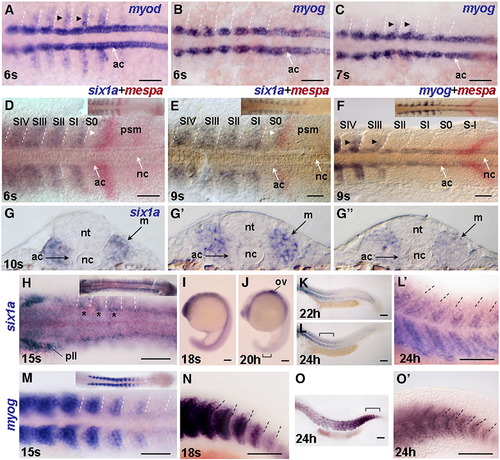
Expression of six1a in the fast muscle precursors initiates before myog expression. Dorsal view of flat mount embryos after WISH for myod at 6 s (A), for myog at 6 s (B), 7 s (C), 15 s (M), for six1a at 15 s (H) and after two-color WISH for six1a (purple) and mespa (red) at 6 s (D) and 9 s (E), and for myog (purple) and mespa (red) at 9 s (F). In all Figures the developmental stage was confirmed by counting somites using DIC (Nomarski) optics. Marking of somites relatively to mespa bands in all Figures was done according to Durbin et al., (2000) unless otherwise stated. Insets in D–F, H, and M show the posterior part of the embryo. Cryostat sections of 10 s embryo after WISH for six1a at the anterior, middle and posterior trunk levels, correspondingly (G, G′, and G″). Lateral view of flat mount embryos after WISH for six1a (I–L, and L′) and myog (N, O, and O′). Whole embryo at 18 s and 20 h (I, J) and posterior body at 22 h and 24 h (K, L). Regions marked by brackets in panels L and O are shown at higher magnification in panels L′ and O′. Bracket in panel J marks weak expression in the tail. Anterior is to the left unless otherwise stated. Dashed lines indicate somite boundaries. Black arrowheads in panels A, C, F indicate expression in fast muscle precursors. Asterisks in panel H mark enhanced six1a expression in the dorsal and rostral part of anterior somites. Note the thin band of six1a expression adjacent to the major mespa band (white arrowhead in panels D and E). Abbreviations: ac, adaxial cells; m, myotome; nc, notochord; nt, neural tube; ov, otic vesicle; pll, posterior lateral line ganglion; psm, presomitic mesoderm. Scale bar, 100 μm. EXPRESSION / LABELING:
|
|
Analysis of efficiency of UM and SM morpholinos. (A–C) UM efficiently blocks EGFP expression encoded by six1a-gfp mRNA in the embryos sequentially injected with six1a-gfp mRNA and UM (60/60 injected with UM and 1/62 injected with CM). Bright field (A–C, left) and fluorescent (A–C, right) images. Embryos were injected with: (A) six1a-gfp mRNA and UM, (B) six1a-gfp mRNA and CM, (C) six1a-gfp mRNA alone and grown until 10 s. (D–F) SM morpholino effectively blocks the nascent splice donor site in six1a pre-mRNA. (D) Semi-quantitative RT-PCR (29 cycles) of six1a mRNA in SM morphants (sm) generated a shorter DNA fragment (asterisk) than in wild-type (wt) control indicative that an aberrant six1a transcript formed in morphants. Note that only the aberrant transcript was detected in SM morphants at 6 s and 10 s. Analysis of β-actin mRNA was done as a control. RT-negative control was carried out simultaneously and no fragment was detected. The primers used in PCR reactions are described (Materials and methods). (E) Sequencing of RT-PCR fragments revealed the “cryptic” splice donor site (arrowhead in panels E and F) located within six1a protein coding sequence and utilized in SM morphants. (F) Aberrant splicing resulted in the deletion of most of the homeodomain helix III and frameshift in the sequence of six1a mRNA. Region of six1a with intron (lower case) involved in splicing is shown on the top. Genomic sequence of six1a was identified by BLAST search in the clone DKEY-225H23 (accession no. BX649231). Sequence of SM morpholino is shown in bold. Deduced amino acid sequences of wild-type Six1a (regular style) and aberrant Six1a (bold style) proteins are shown below the nucleotide sequence. Amino acid residues that are absolutely conserved across homeodomain family of transcription factors are italicized and underlined and the residues that are conservatively substituted are italicized (Banerjee-Basu and Baxevanis, 2001). Asterisk indicates a stop codon in the aberrant six1a mRNA. EXPRESSION / LABELING:
|
|
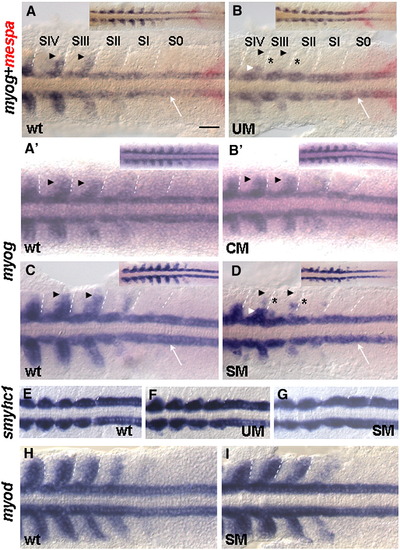
Six1a is required for early myog expression in fast muscle precursors. Flat mount embryos after two-color WISH for myog (purple) and mespa (red) at 9 s (A, B) or single-color in situ hybridization for myog (A′, B′, C, and D), smyhc1 (E–G) or myod (H, I) at 9 s. The four posterior somites with the adjacent presomitic mesoderm are shown. Insets show the posterior part of the embryo. Arrowheads indicate the region of fast muscle precursors. Note that the myog expression is nearly absent along the entire posterior border in SIII and retained in a few medially located cells in SIV (B) and in somites shown in panel D (white arrowheads). The expression in the adaxial cells (white arrow) was retained (B and D). The hardly visible residual myog expression in the affected cells marked by asterisks. CM caused no effect on myog expression (B′). UM or SM cause no obvious effect on the expression of slow muscle-specific marker smyhc1 (F and G, 62/65 and 55/60 of embryos injected with UM or SM, correspondingly) or on myod expression in fast muscle precursors (I; 70/73 of embryos injected with SM). Scale bar, 100 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
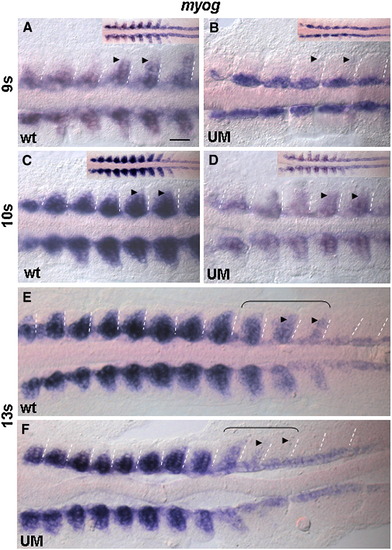
Recovery of myog expression upon initial down-regulation in six1a morphants. Flat mount embryos after WISH for myog. Wild-type embryos (A, C, E) and UM morphants (B, D, F). Arrowheads mark myog expression in fast muscle precursors. Note that the myog expression is nearly absent along the entire posterior border in all somites in morphants at 9 s, increased at 10 s and recovered at 13 s. Also note the delay of recovery in the most posterior somites at 13 s (brackets). Scale bar, 100 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
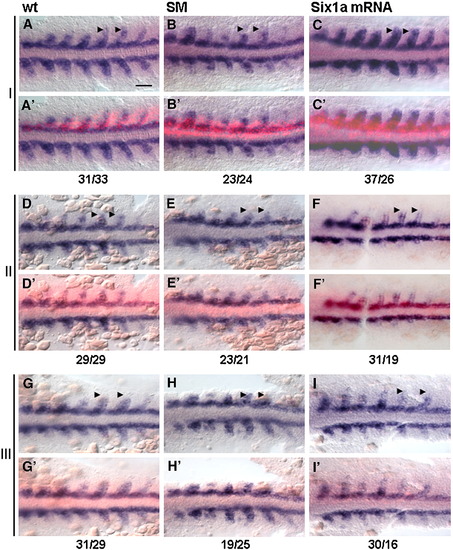
six1a mRNA rescues myog expression in fast muscle precursor region of SM morphants. Flat mounts of embryos after WISH for myog at 9 s. Three sets of embryos from three independent experiments are shown (I–III; n = 3 for each control, morphant and rescue). The bright-field (A, B, C, D, E, F, G, H, and I) and composite bright-field/fluorescent images to visualize Texas Red on injected side (A′, B′, C′, D′, E′, F′, G′, H′, and I′). The uninjected side (lack of red fluorescence) served as an internal control. Opposing pairs of somites were compared. One-sided injection of Texas Red-dextran does not affect myog expression in wild-type (wt, left column). Expression of myog is reduced in the fast muscle precursors in SM morphants with one-sided injection of Texas Red-dextran (SM, middle column). six1a mRNA rescues myog expression in the fast muscle precursor region on the injected side of SM morphants (Six1a mRNA, right column). Arrowheads mark myog expression in fast muscle precursors. Scale bar, 100 μm. Numbers of myog expressing fast cells on the injected side versus the number of cells on the control side of the embryo are shown as a ratio below. EXPRESSION / LABELING:
PHENOTYPE:
|
|
six1a loss-of-function affects the expression of muscle-specific mRNAs in the regions of fast muscle formation. Lateral views of embryos after WISH for myhz1 (A, A′, B, B′), tpma (C, C′, D, and D′), mylz2 (K) at 13 s and for mylz2 at 24 h (L). Anterior somites of control embryos (A, C) and UM morphants (B, D). In panel K, control (wt) and morphant (UM) embryos were placed together for comparison of mylz2 expression levels on the same picture. Three somites indicated by brackets in panels A, B, C, and D are shown at higher magnification in panels A′, B′, C′, and D′, correspondingly. Note that transcripts of tpma and myhz1 genes were excluded from posterior and dorsal regions of somites in morphants (arrowheads in panels B′ and D′). Bright field (E, G) and fluorescent (F, H) images of trunk somites of control embryos and UM morphants of mylz2-gfp transgenic line at 30 h. Some of the melanophores are out of focus due to the slight undulation of the trunk of the morphant (G). Equal numbers of control embryos and UM morphants were grown and half of embryos were collected for RNA isolation. Remaining embryos were grown until 30 h and analyzed. Note the low level of GFP fluorescence in the trunk muscle of morphants. Semiquantitative RT-PCR of gfp mRNA (I) and mylz2 mRNA (J) isolated from uninjected control embryos (wt) and UM morphants (UM) of mylz2-gfp line. Note the weaker band intensity of product amplified from gfp RNA isolated from UM morphants at 24 h. Also, note down-regulation of mylz2 mRNA at 14 h and recovery of expression at 24 h. Down-regulation (K) and recovery (L) of mylz2 mRNA at 13 s and 24 h, correspondingly. Scale bars, 100 μm in panels A, B, C, D, E–H and K; 50 μm in panels A′, B′, C′, and D′; 200 μm in panel L. EXPRESSION / LABELING:
PHENOTYPE:
|
|
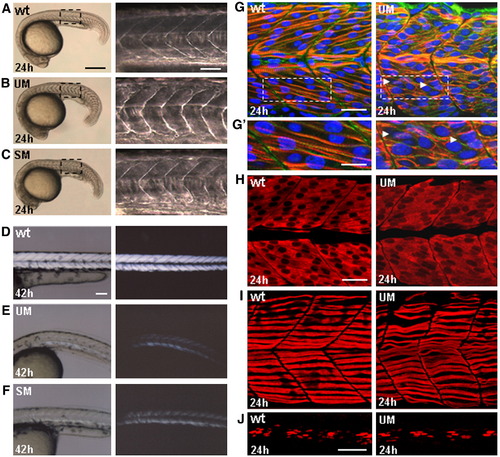
Muscle phenotype of six1a morphants is associated with abnormal differentiation of fast fibers. (A–C) Lateral view of embryos at 24 hpf, (left) and high magnification of boxed regions of the trunk (right). U-shaped somites in the embryos injected with UM or SM (B, C). UM (95/100 injected embryos); SM (91/100 injected embryos). (D–F) Lateral view of embryos at 42 h. Composite bright-field and polarized light (dark-field) images of trunk muscles. Original polarized light images are shown on the right. Note the reduction of muscle birefringence in the embryos injected with UM (E) and SM (F). (G–H) Confocal saggital sections of the trunk muscle at the level of anterior somites 7–9 in control embryos (left) and morphants (right). (G, G′) Immunostaining with the cocktail of mAb A4.1025 (anti-MyHC, red), ab6301 (anti-β-Catenin, green) and DAPI (nuclei, blue). Boxed region of panel G is enlarged in panel G′. Note that the diagonal array of cross-striated fast fibers (red) was not formed and regularity of cellular shapes (green) is absent in morphants. Arrowheads point to the regions with closely positioned nuclei. (H) Staining with F310 (anti-fast MyLC, red). Note the irregular shapes of cells and absence of arrayed organization in morphant fast muscle. (I) Confocal projection of superficial region of myotome after immunostaining with F59 (anti-slow MyHC, red). Note that slow fibers retained their normal position and morphology. (J) Confocal projection of medial region of myotome after immunostaining with 4D9 (anti-Engrailed, red). Scale bars, 300 μm in panels A–C, left; 70 μm in panels A–C, right; 100 μm in panels D–F; 30 μm in panel panel G; 15 μm in panel G′; 30 μm in panels H, I; 50 μm in panel J. |
|
A) Amino acid sequence comparison of zebrafish Six1b with mouse Six1, zebrafish Six2.1 and mouse Six2. Alignment of the deduced amino acid sequences was done using MegAlign program (DNASTAR) with the Clustal W method with an identity weight table. Six domain and homeodomain residues are underlined with thin and thick line, correspondingly. Residues in MSix1, ZSix2.1 and MSix2 that differ from those in Six1b are boxed. Note that there are less amino acid differences between zebrafish Six1b and murine Six1 than between zebrafish Six1b and zebrafish Six2.1. M, mouse; Z, zebrafish. B) Phylogenetic analysis. Phylogenetic trees were constructed as previously described (Bessarab et al., 2004). DSo, Drosophila Sine oculis. The GeneBank accession nos.: DSo, AAB34685; MSix1, AAH23304; ZSix1a, AY466110; ZSix1b, EU109509; ZSix2.1, BAB40699; MSix2, BAA11825; ZSix3a, AAH59414. Note the closer relation of zebrafish Six1b to Six1 homologs than to Six2 homologs. C) Flat mount embryo after WISH for six1b at 24 h. Montage of two images of the same embryo. Dashed line separates ventral (top) and dorsal (bottom) focal planes. Until mid-segmentation, six1b is expressed ubiquitously at low level with slightly elevated expression in the anterior of the embryo. Subsequently, the expression is localized in the ventral epithelium of the otic vesicle (black arrowhead) and in the statoacoustic ganglion (sag). Scale bar, 100 μm. EXPRESSION / LABELING:
|
|
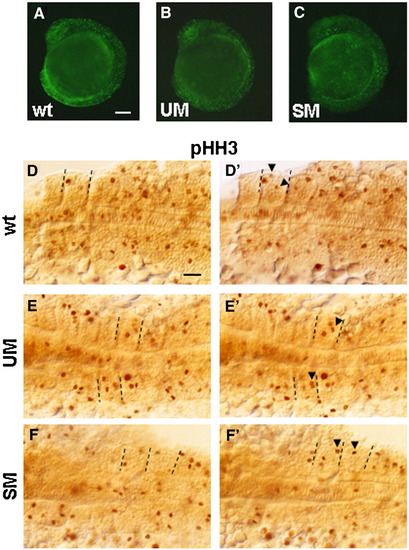
Staining with acridine orange and anti-phosphohistone H3 Ab at 9 s. (A–C) Fluorescent images of control and morphant embryos stained with acridine orange. Dorsal (D, E, F) and ventral (D′, E′, F′) focal planes of flat mounted embryos after immunohistochemistry with anti-phosphohistone H3 Ab. Posterior 6–7 somites are shown. Equal numbers (n = 10) of control embryos and morphants were stained. Arrowheads in D′, E′, F′ indicate phosphohistone H3-positive cells not clearly seen in D, E, F. Dashed lines mark the borders of somites containing these cells. Stained cells were counted in all 9 somites of 3 embryos for each condition (control, UM and SM morphants) and results were summarized in Table 1. No change in phoshohistone-positive cells in notochord was found. Scale bars, 100 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Superficial position of slow fibers in myotome of UM morphants. (A, B) Confocal projections of superficial region of myotome (somites 6, 7, 8) after immunostaining with F59 (anti-slow MyHC). (a′, a″, a″′) and (b′, b″, b″′) y–z sections taken approximately along the plane of the corresponding vertical lines. Scale bar, 30 μm. |

Unillustrated author statements EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 323(2), Bessarab, D.A., Chong, S.W., Srinivas, B.P., and Korzh, V., Six1a is required for the onset of fast muscle differentiation in zebrafish, 216-228, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.