- Title
-
Dynamic formation of microenvironments at the myotendinous junction correlates with muscle fiber morphogenesis in zebrafish
- Authors
- Snow, C.J., and Henry, C.A.
- Source
- Full text @ Gene Expr. Patterns
|
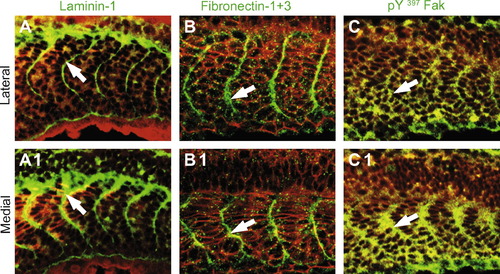
The ECM proteins laminin-1, Fn and Fak concentrate at the initial epithelial somite boundary. All panels are Leica confocal micrographs. All panels side view, anterior left, dorsal top, of 18-somite wild-type embryos. Panels (A–C) are superficial views and panels numbered 1 are medial views of the same Z-stack. β-Catenin which outlines all cells is in red, and laminin, Fn and Fak are each in green. Laminin-1 (A, A1, white arrows), Fn (B, B1, white arrows), and pY397 FAK (C, C1, white arrows) are all concentrated at the somite boundaries throughout the medial-lateral axis of the embryo. EXPRESSION / LABELING:
|
|
The ECM composition of the myoseptal tendon changes during early muscle development. See also Supplemental movies 1 and 2. Laminin persists, whereas Fn is down-regulated, resulting in Fn only concentrating adjacent to slow fibers. All panels were obtained on a Zeiss ApoTome. All Z-series are taken from the transition region of the embryo in which slow-twitch muscle is proceeding laterally. F59, which denotes slow-twitch muscle, is in white. (A) Cartoon summarizing methods. Embryos were stained for slow-twitch muscle (red in cartoons, white in data panels B–G) and Fn (green) or laminin (yellow). Z-series were taken and 3-dimensionaly reconstructed. Transverse views were obtained by rotating the 3-dimensional projection 90 degrees. (B–G) Projections of 20 somite-stage embryos (somite number indicated by S#) stained with F59 to visualize slow muscle (white), Fn (green, the Fn antibody recognizes Fn1 and Fn3 in zebrafish), or laminin (yellow). Lettered panels are side views, anterior left, dorsal top, red arrows denote staining at the myoseptal tendon. Panels numbered 1 are transverse views of the corresponding lettered panel. (D and G) Both Fn and laminin are observed in newly formed somites prior to slow muscle migration. Transverse reconstructions show that Fn and laminin are concentrated both medially (D1, G1, white stars, medial (M) is to the left) and laterally (red stars, lateral is to the right). (C and F) In slightly older somites, Fn is down-regulated medial to migrating slow muscle (C1, medial left, white star medial to the slow fibers shows very little Fn medially). Laminin distribution, however, does not change (F1, white star, laminin is robustly concentrated medially). (B and E) In myotomes, where slow muscle migration is complete, Fn is concentrated adjacent to slow fibers but not fast fibers (B1, white star). Laminin, however, is observed throughout the medial-lateral extent of the myoseptal tendon (E1, white star). (H) Cartoon summarizing dynamic changes in the myoseptal tendon. Slow-twitch fibers (red) are medial in younger somites (posterior, to the right), and laminin (yellow) and Fn (green) are located throughout the medial-lateral extent of the nascent myoseptal tendon. As slow-twitch muscle migrates laterally (in older anterior somites, to the left) Fn is down-regulated at the nascent myoseptal tendon medial to migrating slow-twitch fibers. Scale bar: 50 μm. EXPRESSION / LABELING:
|
|
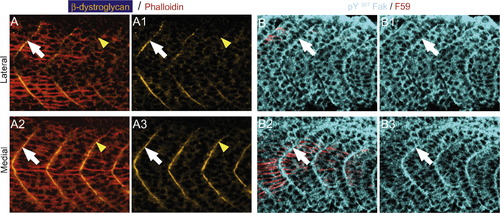
β-Dystroglycan and pY397 FAK concentrate at the myoseptal tendon medial to migrating slow muscle fibers. All panels are ApoTome images, side views, anterior left, dorsal top, of the transition region, where slow muscle is migrating, in 19–21 somite embryos. Phalloidin, denoting actin, is red in panel A and F59, denotes slow-twitch muscle in red for panel B. Panels numbered 1 and 3 show only β-dystroglycan and pY397 FAK, respectively. (A) Lateral to migrating slow fibers, β-dystroglycan (green) does not concentrate at the boundary (A, A1, yellow arrowheads) but does concentrate slightly anteriorly where slow-twitch muscle has migrated (white arrows, A, A1). β-Dystroglycan concentrates at the boundary medial to migrating slow fibers (A2, A3, note robust concentration adjacent to yellow arrowheads where β-dystroglycan was not concentrated laterally). (B) Similarly, pY397 FAK concentration at the boundary is increased medial and adjacent to slow muscle fibers (B2, B3, white arrowheads). EXPRESSION / LABELING:
|
|
Slow-twitch muscle specification and subsequent migration is not required for localization of laminin, Fak and Fn at the MTJ during muscle morphogenesis, however expression is sometimes disrupted. All panels were obtained using a Zeiss ApoTome. Side views, anterior left, dorsal top of 24 hpf embryos. (A) Laminin (yellow) is highly concentrated at the MTJ of wild-type embryos (A, white arrow). In smu embryos, there are frequently gaps in the myotome boundary, and laminin often congregates at these gaps (A1, arrowheads) in addition to the MTJ (A1, arrow). (B) pY397 FAK (blue) is concentrated at the MTJ of wild-type embryos (B, white arrow). In smu embryos, pY397 FAK concentrates at the MTJ (B1, white arrow) but not at gaps (B1, yellow arrowheads). (C) Fn (green) is concentrated at the lateral MTJ of wild-type embryos (C, white arrow). In smu embryos, Fn concentrates at the MTJ (C1, white arrow) but is sometimes reduced/absent (C1, green arrowhead). |
Reprinted from Gene expression patterns : GEP, 9(1), Snow, C.J., and Henry, C.A., Dynamic formation of microenvironments at the myotendinous junction correlates with muscle fiber morphogenesis in zebrafish, 37-42, Copyright (2009) with permission from Elsevier. Full text @ Gene Expr. Patterns