- Title
-
Lpp is involved in Wnt/PCP signaling and acts together with Scrib to mediate convergence and extension movements during zebrafish gastrulation
- Authors
- Vervenne, H.B., Crombez, K.R., Lambaerts, K., Carvalho, L., Koeppen, M., Heisenberg, C.P., Van de Ven, W.J., and Petit, M.M.
- Source
- Full text @ Dev. Biol.
|
Expression of lpp during zebrafish development. (A) Expression of lpp and β-actin mRNA as determined by RT-PCR at different stages of zebrafish development and in non-fertilized eggs (NF). nc, negative control. (B–N) Whole-mount in situ hybridization analyses for zebrafish lpp expression at different stages of embryonic development using an RNA probe against the 3′UTR of lpp. (B) 16-cell stage, animal pole view. (C) dome stage, animal pole view. (D) shield stage, animal pole view, dorsal to the right. (E) tailbud stage, lateral view, dorsal to the right. (F) tailbud stage, dorsal view, anterior on top. (G) tailbud stage, vegetal pole view. (H) 14-somite stage, lateral view, dorsal to the right. (I) 14-somite stage, dorsal view. (J) Prim-5 stage, head region, dorsal view. (K) Prim-5 stage, lateral view. (L) Prim-5 stage, dorsal view. (M) Long-pec stage, lateral view. (N) Long-pec stage, frontal view. Abbreviations: nc, notochord; tb, tailbud; isv, intersomitic vessels; h, heart. |
|
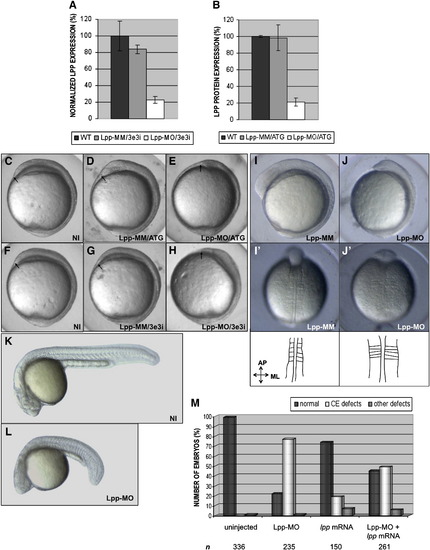
Knockdown of lpp disrupts convergence and extension movements. (A, B) Validation of the morpholinos: (A) quantitative RT-PCR using total cDNA from tailbud stage embryos as a template, shows an 80% reduction of lpp expression after injection of Lpp-MO/3e3i but not after injection of Lpp-MM/3e3i; (B) Lpp protein levels of prim-5 stage embryos are strongly reduced after injection of Lpp-MO/ATG but not after injection of Lpp-MM/ATG, as shown by quantification of the intensities of the protein bands after Western blot analysis (cumulative results of three experiments). Error bars represent standard errors of the mean. (C–L) Morpholinos (Lpp-MO/ATG and Lpp-MO/3e3i) or mismatch control morpholinos (Lpp-MM/ATG and Lpp-MM/3e3i) were injected at the one- to two-cell stage and morphology was assessed at different stages. (C–H) tailbud stage, lateral view, dorsal to the right; the anterior-most structure is indicated with an arrow. (I, J) 5-somite stage, lateral view, dorsal to the right. (I′, J′) 5-somite stage, dorsal view; drawings schematically show the notochord and somites of the embryos in the pictures. (K, L) prim-5 stage, lateral view, anterior to the left. Abbreviations: NI, non-injected; ML, mediolateral axis; AP, anterior–posterior axis. (M) Zebrafish embryos were injected with Lpp-MO/3e3i (5 ng) or synthetic mRNA (7.5 pg) encoding full-length Lpp, or were co-injected with Lpp-MO/3e3i (5 ng) and synthetic lpp mRNA (7.5 pg). At tailbud stage, the embryos were scored morphologically for the presence of C&E defects, as depicted in panels E and H. The phenotypes of the embryos of three independent experiments were scored and the percentages of normal embryos, embryos with C&E defects (“CE defects”) and embryos with defects other than C&E defects (“other defects”) are indicated. n, total number of embryos injected for each condition. |
|
Expression of marker genes in lpp morphants. Wild-type embryos and embryos injected with Lpp-morpholino were analyzed for the expression domains of marker genes by whole mount in situ hybridization. In each section (A–F), wild-type (WT) embryos are depicted on the left and lpp morphants (Lpp-MO) on the right. (A) gsc, shield stage, animal pole view, dorsal to the right. (B, B′) ntl (Δ), dlx-3 (∗) and hgg1 (O), tailbud stage, dorsal views (B) and animal pole views (B′) of the same embryo. (C) myoD, tailbud stage, dorsal view. (D) papc, two-somite stage, dorsal view. (E) chordin, shield stage, dorsal view. (F) bmp4, shield stage, animal pole view, dorsal to the right. |
|
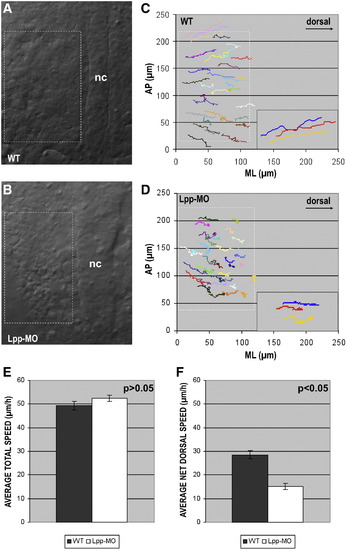
Lpp function is required for efficient dorsal convergence. Dorsal views of (A) wild-type (WT) and (B) Lpp-MO/ATG (5 ng) injected embryos at tailbud stage (boxes highlight the paraxial region, which was the focus of the migration analysis). (C, D) Cell tracks of paraxial cells from (C) wild-type and (D) Lpp-MO/ATG injected embryos extracted from Normarski time-lapse images. Each graph shows representative data obtained from individual embryos. Insets are enlarged tracks of three representative cells. AP, anterior–posterior axis; ML, mediolateral axis. (E) No significant (p = 0.19) difference in total speed (μm/h) could be detected between wild-type and Lpp-MO/ATG injected embryos, (F) whereas net dorsal speed after Lpp-MO/ATG injection was significantly (p = 1.1 x 10- 8) reduced. (E, F) Graphs represent average data from 60 cells from 2 embryos for each condition; error bars represent standard errors of the mean. PHENOTYPE:
|
|
lpp mRNA levels are reduced in Wnt11 morphants and in embryos overexpressing Wnt11 or a dominant negative form of Rho kinase 2. Zebrafish embryos were injected with Wnt11-MO (2 ng), wnt11 RNA (100 pg) or dnRok2 RNA (100 pg). Embryos showing clear C&E defects at tailbud stage were selected and total RNA was extracted. Quantitative RT-PCR with primers for lpp showed reduced levels of lpp mRNA in all three conditions (*, p < 0.05; **, p < 0.01). n, total number of embryos per condition from four independent experiments. EXPRESSION / LABELING:
|
|
lpp genetically interacts with scrib and trilobite for the regulation of C&E during zebrafish gastrulation. (A) GST fusion proteins, as indicated, and GST alone were expressed in E. coli, purified and analyzed by SDS-PAGE and GelCode Blue Stain Reagent staining. All proteins were expressed well. Protein markers are as indicated. (B) All four PDZ domains of wild-type zebrafish Scrib (AA 700–1517) were synthesized in vitro and 35S-labeled. Labeled proteins were incubated with immobilized GST, GST-Lpp-LTWT or GST-Lpp-LTL557A and allowed to interact overnight at 4 °C. Bound proteins were eluted in sample buffer, separated by SDS-PAGE and visualized by autoradiography. The amount of synthesized protein loaded as a reference on the gel corresponds to 10% of the input used in each binding experiment. (C) Suboptimal doses (s.o.) of Lpp-MO/ATG (1 ng) or Scrib-MO (1 ng) were injected separately or were co-injected in the one- to two-cell stage; the phenotypes were scored at the five-somite stage and compared to embryos injected with optimal doses (o.) of either of the morpholinos (Lpp-MO, 5 ng; Scrib-MO, 5 ng). Phenotypes scored were: normal, C&E defects, and defects other than C&E defects (“other defects”). (D) Suboptimal doses (s.o.) of Lpp-MO/ATG (1 ng) or Tri-MO (0.25 ng) were injected separately or were co-injected in the one- to two-cell stage. The phenotypes were scored at 11 hpf and compared to embryos injected with optimal doses (o.) of either of the morpholinos (Lpp-MO, 5 ng; Tri-MO, 4 ng). Phenotypes scored were: normal or mild C&E defects (C&E+/-), moderate (C&E+) or strong (C&E++) C&E defects, and defects other than C&E defects (“other defects”). Pictures in panels C and D show for each condition embryos representative for the phenotype of the largest group(s). Abbreviations: NI, uninjected. n, total number of embryos per condition from two independent experiments. PHENOTYPE:
|
Reprinted from Developmental Biology, 320(1), Vervenne, H.B., Crombez, K.R., Lambaerts, K., Carvalho, L., Koeppen, M., Heisenberg, C.P., Van de Ven, W.J., and Petit, M.M., Lpp is involved in Wnt/PCP signaling and acts together with Scrib to mediate convergence and extension movements during zebrafish gastrulation, 267-277, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.