- Title
-
Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish
- Authors
- Holzschuh, J., Wada, N., Wada, C., Schaffer, A., Javidan, Y., Tallafuss, A., Bally-Cuif, L., and Schilling, T.F.
- Source
- Full text @ Development
|
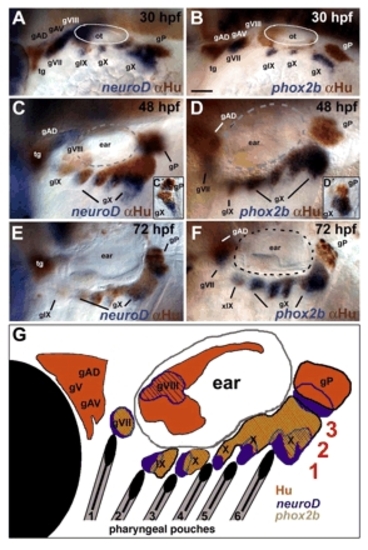
Molecular markers of neurogenesis reveal heterogeneity within the epibranchial ganglia. Lateral views, anterior to the left, showing the pattern of dorsolateral and epibranchial ganglia at 30 (A,B), 48 (C,D) and 72 (E,F) hpf. Insets show vibratome sections of epibranchial ganglia from neurod/α-Hu (C′) and phox2b/α-Hu (D′) double-stained 48 hpf embryos. (A,C,C′,E) In situ hybridization to detect neurod mRNA in newly specified neural progenitors (blue), partially overlaps with α-Hu staining in differentiating neurons (brown). (B,D,D′,F) Most neural progenitors co-express phox2b at 30 hpf (B) but expression becomes restricted to a more proximal populations by 48 and 72 hpf (D,F). (G) Diagram indicating the spatial relationships of pharyngeal pouches (1-6, black), and three domains of gene expression within the ganglia (1-3, red). Hu+ proximal neural progenitors are shown in orange, phox2b+ cells in green (overlap with Hu is indicated as diagonal hatching) and more distal neurod+ cells in blue. gAD, dorsal anterior lateral line; gAV, ventral anterior lateral line; gP, posterior lateral line; gV, trigeminal; gVII, facial (geniculate), gVIII, auditory; gIX, glossopharyngeal (petrosal); gX, vagal (nodose); ot, otic placode; tg, trigeminal. Scale bar: 50 μm. EXPRESSION / LABELING:
|
|
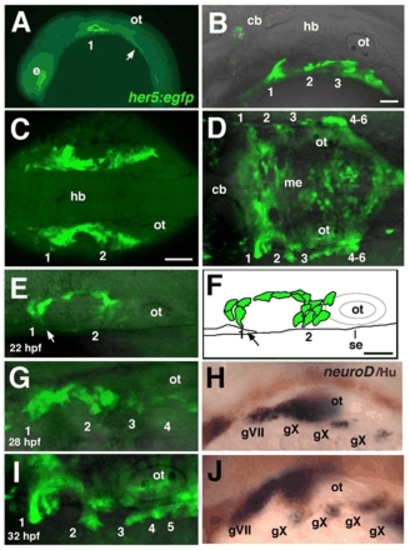
Pharyngeal pouch formation coincides with epibranchial neurogenesis. (A-E,G,I) Confocal images of live, homozygous her5:egfp transgenic embryos; anterior to the left. (A) 16 hpf embryo, lateral view; pouch 1, as well as an unsegmented endoderm located further posteriorly (arrow), is labeled. (B) 26 hpf embryo, lateral view; pouches 1-3 are labeled. (C) 18 hpf embryo, dorsal view; pouches 1 and 2 on each side are joined along the AP axis by a continuous band of endoderm surrounding mesenchyme of the second arch. (D) 32 hpf embryo, dorsal view; pouches (1-6) form only in the most lateral endoderm, while her5:egfp also labels a flattened layer of medial endoderm (me). (E,G,I) Sequence of confocal images of her5:egfp, in dorsal view, at 22 (E), 28 (F) and 32 (H) hpf. (E) 22 hpf embryo; pouch-forming endodermal cells extend filopodial processes laterally (arrow). (F) An illustration of the embryo shown in E. Arrow indicates movement of GFP+ cells toward the surface ectoderm (se). (G-J) Embryos analyzed for her5:egfp expression at 28 (G) and 32 (I) hpf were fixed and subjected to in situ hybridization (H,J) for neurod (blue) combined with immunolabeling with α-Hu antibody (brown). 1-6, pharyngeal pouches; cb, cerebellum; e, eye; gV, trigeminal ganglion; gVII, facial (geniculate) ganglion; gIX, glossopharyngeal (petrosal) ganglion; gX, vagal (nodose) ganglion; hb, hindbrain; me, medial endoderm; ot, otic vesicle. Scale bars: 50 μm; in B for A,B; in C for C,D; in F for E-J. |
|
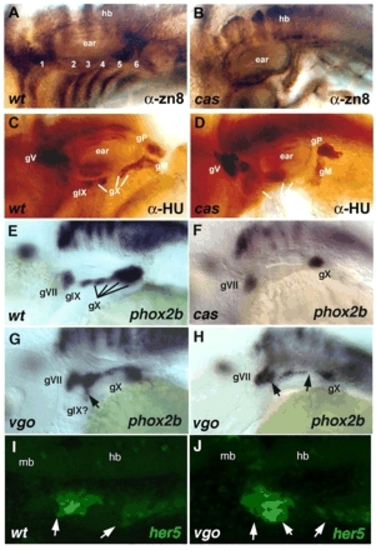
Epibranchial defects in mutants that disrupt endoderm. Lateral views, anterior to the left. (A,B) Wild type and cas mutant at 48 hpf, immunolabeled with anti-zn8 antibody, which labels pharyngeal pouches 1-6. (C,D) Wild type and cas mutant at 48 hpf, immunolabeled with anti-Hu to mark sensory neurons. (E,F) Wild type and cas mutant at 48 hpf labeled by whole-mount in situ hybridization for phox2b mRNA in neural progenitors. (G,H) Examples of phox2b expression in vgo mutants. Arrow in G denotes a ventrally extended ganglion; arrows in H denote fused or missing ganglia. (I,J) her5:egfp expression in living wild type and vgo mutant at 24 hpf. Arrows in I denote contacts between pouches and ectoderm; arrows in J denote deformed or missing pouches. 1-5, pharyngeal pouches; gM, medial portion of vagal; gP, posterior lateral line; gV, trigeminal; gIX, glossopharyngeal; gX, vagal; hb, hindbrain; mb, midbrain. |
|
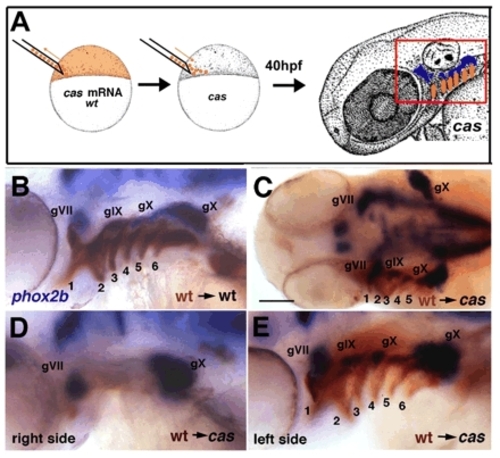
Restoration of endoderm rescues epibranchial ganglia in cas/sox32 mutants. (A) Diagram showing transplantation technique. Cells were grafted at blastula stages from cas mRNA-injected (or TaramA-injected, tar*), wild-type (wt) donors co-injected with biotinylated-dextran (orange) into unlabeled cas mutant hosts. Transplants gave rise to endodermal cells in the arches (box outlined in red) and induced epibranchial ganglia (blue). (B,D,E) Lateral views, 48 hpf, showing examples of epibranchial rescue by such cas-injected endodermal transplants (brown), as determined by in situ hybridization for phox2b expression (blue). pf, pectoral fin. (C) Dorsal view. Scale bar: 50 μm. |
|
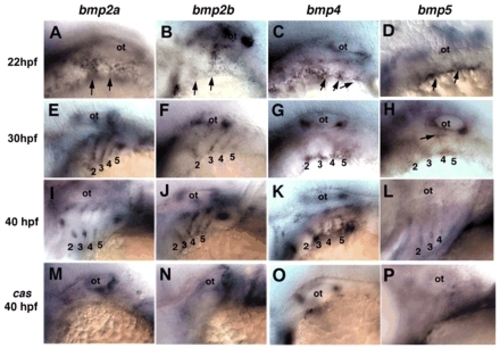
BMP gene expression in the pharyngeal pouches during epibranchial development, and defects in cas mutants. Lateral views, anterior to the left. Whole-mount in situ hybridization was performed in wild types at 22 hpf (top row), 30 hpf (second row) and 40 hpf (third row), and in cas mutants at 40 hpf (bottom row), for bmp2a (A,E,I,M), bmp2b (B,F,J,N), bmp4 (C,G,K,O) and bmp5 (D,H,L,P). Arrows indicate initial expression at the ventrolateral edges of each pouch. Arrow in H denotes bmp5 expression in the dorsal pouches. 1-5, pharyngeal pouches; ot, otic capsule. EXPRESSION / LABELING:
|
|
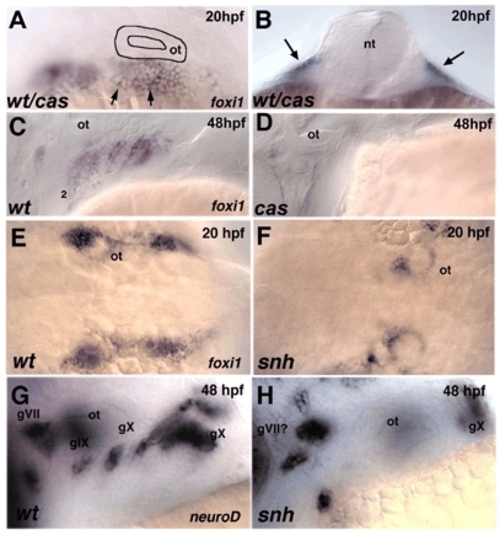
Early placodal defects in cas/sox32 and snh/bmp7 mutants. Lateral views, anterior to the left except for in B, which is a transverse section, and E,F, which are dorsal views. (A,B) Whole-mount in situ hybridization for foxi1 in embryos at 20 hpf from a cross between cas+/– heterozygotes reveals no difference between wild types and mutants. Arrows in B indicate ectodermal expression. (C,D) foxi1 expression at 48 hpf in both pouch endoderm and epibranchial placodes in wild type, and loss of expression in cas mutants. (E,F) Dorsal views showing foxi1 expression at 20 hpf in wild type and severe reduction of expression in snh/bmp7 mutants. (G,H) Lateral views showing neurod expression in epibranchial ganglia and severe reductions in snh/bmp7 mutants. 1-6, pharyngeal pouches; gVII, facial; gIX, glossopharyngeal; gX, vagal; nt, neural tube; ot, otic vesicle. EXPRESSION / LABELING:
|
|
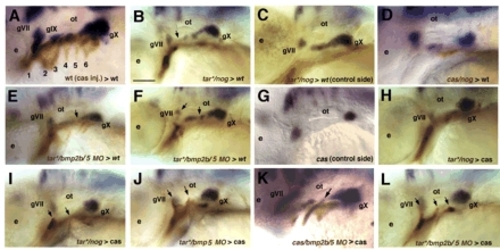
BMP signaling is required locally in endoderm for epibranchial neurogenesis. Lateral views, anterior to the left. In situ hybridization for phox2b mRNA labels epibranchials at 48 hpf in all panels, whereas biotinylated-dextran injected into the donor labels transplanted cells (brown). (A) Wild-type pattern of phox2b expression in a control embryo that received a large graft of cas-injected endodermal cells. Similar results were obtained with tar*. (B-F) Wild-type hosts that received transplants of donor cells co-injected with tar*/dextran and either nog mRNA (B-D) or bmp2b and bmp5 MOs (E,F), which disrupt epibranchial formation (arrows). (G) phox2b expression in a cas mutant showing lack of epibranchials at 48 hpf. (H-L) Transplantation of tar*/nog (H,I) tar*/bmp5MO (J), cas/bmp2b/5MO-injected (K) or tar*/bmp2b/5MO-injected (L) endodermal pouches (arrows) fails to rescue epibranchials in cas mutants. Scale bar: 50 μm. |
|
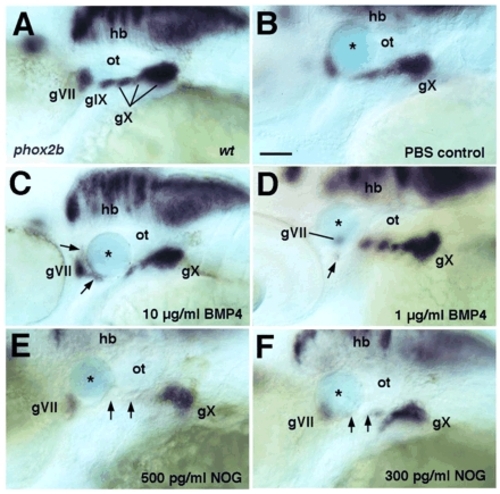
BMP4-coated beads induce, and NOG-coated beads inhibit, epibranchial formation. Lateral views, anterior to the left. In situ hybridization for phox2b mRNA to label epibranchials at 48 hpf. (A) Wild-type control embryo. (B-F) Embryos that received Affi-Gel Blue beads soaked in either PBS (B), BMP4 (10 µg/ml, C; 1 µg/ml, D) or NOG protein (500 pg/ml, E; 300 pg/ml, F). Arrows in C and D indicate ectopic phox2b+ cells adjacent to the bead; arrows in E and F denote missing ganglia adjacent to the NOG-coated bead. Asterisks indicate beads. gVII, facial; gIX, glossopharyngeal; gX, vagal; ot, otic vesicle. Scale bar: 50 μm. EXPRESSION / LABELING:
|

Unillustrated author statements EXPRESSION / LABELING:
|