- Title
-
Zebrafish periostin is required for the adhesion of muscle fiber bundles to the myoseptum and for the differentiation of muscle fibers
- Authors
- Kudo, H., Amizuka, N., Araki, K., Inohaya, K., and Kudo A.
- Source
- Full text @ Dev. Biol.
|
Expression of the periostin gene during embryogenesis. Wild-type embryos were hybridized with the periostin (A, B, D–I, and K–P) or myoD (C, J, and L) probe and fss embryos, with the periostin probe (H). The orientation is anterior at the top (A–E) and anterior at the left (F–H and K–P). No periostin expression is detected at the bud stage (A), and the segmented expression of periostin (B, D, and E) or myoD (C) is observed from the dorsal view of the 8-somite (B and C) and 16-somite stages (E) and from the lateral view of the 16-somite stage (D). A transverse section (I) and a sagittal section (K) show the expression of periostin in the lateral (I) and rostral (K) parts of segmented somites; and another transverse section shows myoD expression in the lateral and adaxial parts (arrows, see also I) of the somites (J). In flat preparation of 10-somite embryos (L), double labeling with periostin (purple, arrowheads) and myoD (red) shows their expressions, which lined one after the other in newly formed (posterior) somites but ambiguously in already formed (anterior) somites. In the lateral view of the 20-somite (F) or 26-hpf (G) stage, widely spread expression of periostin is observed. In fss embryos, no metameric pattern is observed in the trunk region (H). Horizontal (M and N) and sagittal (O and P) sections of 36-hpf embryos show that periostin expression is restricted to the small triangular regions that are surrounded by adjacent somites and notochord or spinal cord (N and P). Abbreviations: NC, notochord; S, somite; SC, spinal cord. Scale bars: A–H, 100 μm; I–P, 25 μm. |
|
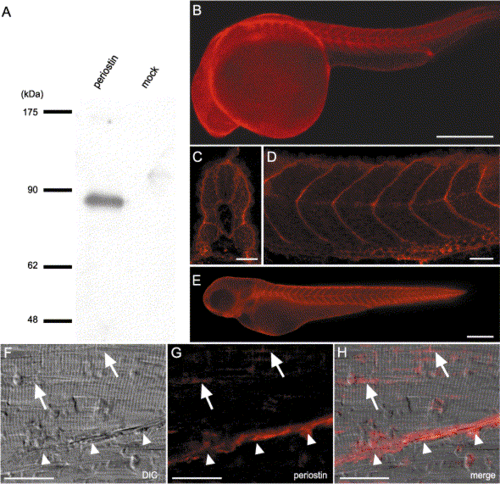
Localization of periostin protein in embryos and adult muscles. (A) Western blot analysis of the conditioned medium from supernatants of periostin transfectants or mock transfectants by using antiperiostin antibody (anti-CTR18). An immunoreactive band of 86 kDa is observed in the lane of periostin transfectants, which size corresponds to the calculated molecular weight of 85,884. (B–F) Whole-mount immunostaining with the anti-periostin antibody. Periostin staining of 24-hpf embryos (B–D) in transverse (C) and sagittal (D) sections of the trunk reveals the localization of periostin in the transverse myosepta and the periphery of myotomes, respectively. In 48-hpf embryos (E), periostin localization in the transverse myosepta is maintained. (F, G, and H) Immunohistochemistry of skeletal muscle of 9-month-old zebrafish with cryosectioning. DIC microscopic image reveals that striated muscle fibers and a myoseptum (white arrowheads) diagonally attach each other (F). Periostin immunoreactivities are present in the myoseptum (white arrowheads) and adjoining sites among the muscle bundles (G, H, and white arrows). (H) Merged image of DIC and fluorescent images. Abbreviation; DIC, differential interference contrast microscope. Scale bars: B and E, 250 μm; C, D, and F–H, 25 μm. |
|
Immunoelectron microscopic image of periostin in myoseptum of 48-hpf embryos. Myofibrils and myoseptum are obliquely positioned with respect to each other. Periostin immunoreactivity (black color, arrowheads) is observed along the surface of the myoseptum. In addition, the immunoreactivity is concentrated at the intersection of myosepta and extended lines of myofibers. Abbreviations: MF, myofibers; MS, myoseptum. Scale bar: 5 μm. |
|
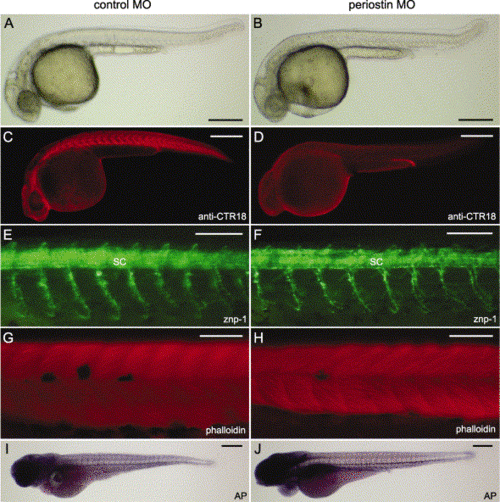
Characterization of the periostin morphant phenotype. Control MO (A, C, E, G, and I) and periostin MO (B, D, F, H, and J) -injected embryos are shown with the anterior aspect at the left and the dorsal one at the top. (A an B) Live images show morphological phenotypes at 33 hpf. (C and D) Whole-mount immunostaining with the anti-periostin antibody (anti-CTR18) indicates the loss of periostin at the transverse myosepta in the 27-hpf periostin morphants (D). (E and F) Whole-mount immunostaining with znp-1 reveals the primary motor axons of 30-hpf embryos. (G and H) Alexa Fluor 568-phalloidin staining of 48-hpf embryos shows somitic muscles of the midtrunk region. (I and J) Endogenous alkaline phosphatase activities reveal metameric patterns of the intersomitic vessels in 72-hpf embryos. Abbreviations: AP, alkaline phosphatase; MO, morpholino antisense oligonucleotide; SC, spinal cord. Scale bars: A–D, I, and J, 250 μm; E–H, 100 μm. |
|
Histological analysis of periostin morphants. Sagittal sections of control MO (A and C)- and periostin MO (B and D)-injected embryos at 24 hpf (A and B) and 48 hpf (C and D). Immature myofibers extend the entire length of the somites in control MO-injected embryos (A, black arrows), whereas they are disrupted at irregular intervals in periostin morphants (B, white arrows). In 48-hpf control MO-injected embryos, striated myofibers occupy the somites (C). In 48-hpf periostin morphants, the myoseptum is partly disrupted (D, white arrowheads) and striated myofibrils stretch across the two-somite length. In the ventral part of the myotome, the periostin morphants show less differentiated muscle fibers than the control morphants (D, asterisk, vs. asterisk in C). Abbreviation: NC, notochord. Scale bar: 15 μm. |
|
Structure of the transverse myosepta in wild-type embryos at 48 hpf. The triangular regions surrounded by adjacent myotomes and notochord or spinal cord. Myosepta are recognized as cytoplasmic processes (arrows in A and B), which are extended from cells in the triangular regions; and these cytoplasmic processes have small secondary protrusions (arrowheads in B). Cell membranes (an arrow in C) in the cytoplasmic process of myoseptum can be obviously discernible. Abbreviations: M, myotome; MF, myofibers; N, nucleus; NC, notochord; V, blood vessel. Scale bars: A, 6 μm; B, 1.5 μm; C, 0.7 μm. |
|
Electron micrographs of sagittal sections through somites of control MO (A, C, E, and F) and periostin MO (B, D, G, and H)-injected embryos at 48 hpf. The myotomes of control MO-injected embryos are occupied with striated muscle fibers (A and E), and ordered staircase-like structures are seen at the place where a myoseptum and myofibers contact one another (C, arrowheads), whereas the triangular areas are not filled with actin–myosin filaments (arrowheads in C and asterisk in F). Higher magnification of the area boxed (E) shows small protrusions that extend from the myosepta and a triangular area occupied by granular substances (F, asterisk). Conversely, in myotomes of periostin morphants, immature myoblasts are included (B), and striated myofibers of various widths show more frequent branching and merging (D). Moreover, the staircase-like structure is not observed (arrows in D), and the myoseptum is partly disrupted (arrows in G). At the contact points between a myoseptum and myofibers, fibrils are appeared in the whole triangular area and they connect the myoseptum with the z-discs (arrows in H). Scale bars: A and B, 6 μm; C and D, 3 μm; E and G, 4 μm; F and H, 0.7 μm. |
Reprinted from Developmental Biology, 267(2), Kudo, H., Amizuka, N., Araki, K., Inohaya, K., and Kudo A., Zebrafish periostin is required for the adhesion of muscle fiber bundles to the myoseptum and for the differentiation of muscle fibers, 473-487, Copyright (2004) with permission from Elsevier. Full text @ Dev. Biol.