Fig. 6
|
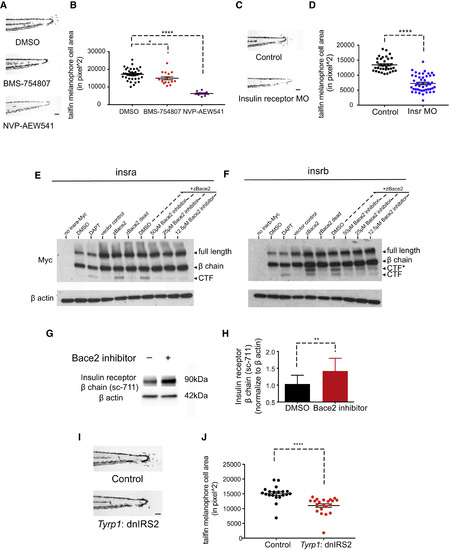
The Insulin Receptor Is a Substrate for Bace2 and Regulates Melanophore Dendricity (A and B) Inhibition of the insulin receptor in bace2−/− embryos using the insulin receptor inhibitors BMS-754807 (7.5 μM) and NVP-AEW541 (60 μM) rescue the hyperdendritic melanophores in the mutant, quantified in (B). bace2−/− embryos are treated from 24 to 72 hpf, and the data are from three independent experiments. One-way ANOVA followed by Holm-Sidak's multiple comparisons test, ∗p < 0.1, ∗∗∗∗p < 0.0001. (C and D) Morpholino knockdown of insulin receptor (Insr) in bace2−/− embryos rescues the hyperdendritic melanophores, quantified in (D). Insra and insrb morpholinos were coinjected into bace2−/− embryos to deplete all insulin receptors. The data are from three independent experiments, two-tailed t test, ∗∗∗∗p < 0.0001. (E and F) Bace2 cleaves the insulin receptor. Expression of zebrafish Insra-Myc (E) and Insrb-Myc (F) fusion protein in HEK293T cells give rise to full-length insulin receptor and the β chain. Inhibition of γ secretase using DAPT (2 μM, 24-hr treatment) prevents the CTF from being cleaved, leading to CTF accumulation. Expression of zebrafish Bace2 (zBace2) on top of the Insra-Myc fusion protein leads to production of single CTF band (E). zBace2 addition leads to the production of two fragments from Insrb-Myc, one that migrates similarly to the CTF and additional larger fragment designated as CTF∗ (F). This is not seen with the enzymatic dead version of Bace2 (zBace2 dead) or after Bace2 inhibition with PF-06663195 (24-hr treatment). zBace2 dead was constructed by site-mutagenesis of two conserved catalytic sites aspartic acids into alanines (D98A and D292A). (G and H) Bace2 inhibition with PF-06663195 (25 μM, 24-hr treatment) in ZMEL1 cells, which normally express both Bace2 and the insulin receptor, leads to accumulation of endogenous insulin receptor β chain, quantified in (H). The data are from five independent experiments, two-tailed t test, ∗∗p < 0.01. (I and J) Melanophore-specific inhibition of insulin signaling in bace2−/− mutants rescues melanophores hyperdendricity in the tailfin. A mosaic F0 transgenic line was created in which the fugu tyrp1 promoter drives dominant-negative insulin receptor substrate 2 (dnIRS2) fused to EGFP in the melanophores (along with a cmlc2: mCherry transgene marker). At 72 hpf, tailfin melanophore cell area was quantified in uninjected control (Control) and transgene-positive injected embryos (J). The data are from three independent experiments, two-tailed t test, ∗∗∗∗p < 0.0001. All bar graphs are presented as means ± SEM. Scale bar, 100 μm. See also Figures S5 and S6. |
| Fish: | |
|---|---|
| Condition: | |
| Knockdown Reagents: | |
| Observed In: | |
| Stage: | Protruding-mouth |
Reprinted from Developmental Cell, 45(5), Zhang, Y.M., Zimmer, M.A., Guardia, T., Callahan, S.J., Mondal, C., Di Martino, J., Takagi, T., Fennell, M., Garippa, R., Campbell, N.R., Bravo-Cordero, J.J., White, R.M., Distant Insulin Signaling Regulates Vertebrate Pigmentation through the Sheddase Bace2, 580-594.e7, Copyright (2018) with permission from Elsevier. Full text @ Dev. Cell