Fig. 4
- ID
- ZDB-FIG-120216-20
- Publication
- Mishima et al., 2012 - Translational inhibition by deadenylation-independent mechanisms is central to microRNA-mediated silencing in zebrafish
- Other Figures
- All Figure Page
- Back to All Figure Page
|
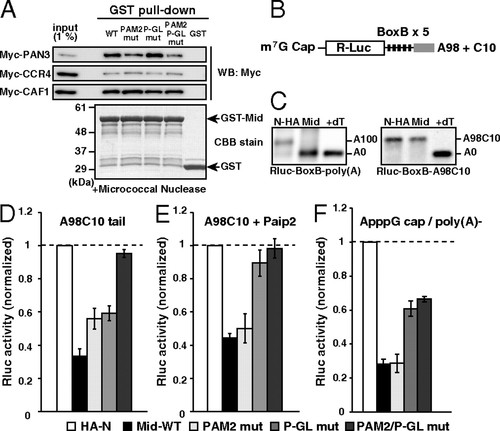
The Mid domain of TNRC6A represses translation via deadenylation-independent mechanisms. (A) The GST-pulldown assay detecting interactions between the GST-Mid domain and deadenylase components translated in rabbit reticulocyte lysate. Total of 1% of in vitro translation reaction was loaded as an input. Myc-tagged proteins were detected using Western blotting (Upper). GST fusion proteins were visualized using CBB stain (Lower). (B) Rluc reporter mRNA containing 5 copies of BoxB sites followed by the A98C10 tail. (C) The poly(A) tail analysis of the Rluc-BoxB reporter mRNAs at six hours, in the presence of control HA-λN peptide (HA-N) or the HA-λN tagged Mid domain (Mid). Left: The reporter mRNA with a normal poly(A) tail [Rluc-BoxB-poly(A)]. Right: The reporter mRNA with an A98C10 tail (Rluc-BoxB-A98C10). The lane +dT shows a completely deadenylated fragment (A0). (D) Tethering assay of the TNRC6A Mid domain with reporter mRNA containing the A98C10 tail in the presence of Myc-GFP. (E) Tethering assay of the TNRC6A Mid domain with reporter mRNA containing the A98C10 tail in the presence of Myc-Paip2. (F) Tethering assay of the TNRC6A Mid domain constructs with a reporter mRNA containing the 52 ApppG cap without a poly(A) tail. Graphs in D, E, and F show the averages of three independent experiments. Error bars show SD. |