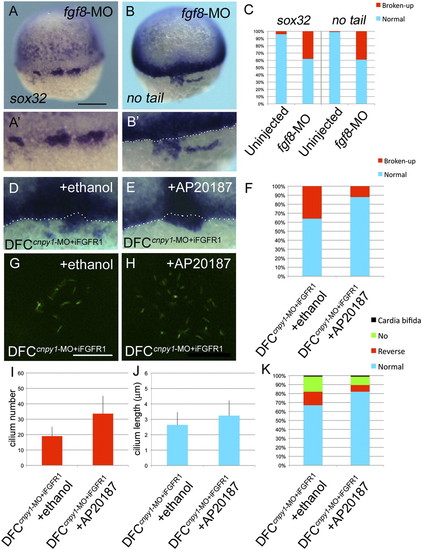
FGF signaling plays crucial roles in DFC clustering and KV ciliogenesis. (A and B) sox32 (A) or no tail (B) expression in fgf8-MO–injected embryos. Dorsal view, anterior to the top. (Scale bar: 200 μm.) (A2 and B2) Higher-magnification images highlight DFCs. (B2, D, and E) The white dotted lines mark the boundary between DFCs and the blastoderm margin. (C) Percentages of normal or broken-up DFCs were scored by using the sox32 or no tail expression patterns in uninjected (n = 68 or 89) or fgf8-MO (n = 61 or 69) embryos. Statistically significant (P < 0.05) differences could be seen in uninjected versus fgf8-MO embryos. (D–K) Transient activation of FGF signaling restored the broken-up DFC phenotype (D–F), ciliogenesis (G–J), and cardiac laterality (K) in DFCcnpy1-MO embryos. (D and E) Expression of no tail in DFCcnpy1-MO+iFGFR1 embryos treated with ethanol (D) or AP20187 (E). (F) Percentages of broken-up DFC phenotype in ethanol-treated (n = 84) or AP20187-treated (n = 93) DFCcnpy1-MO+iFGFR1 embryos. The conditional activation of Fgfr1 after treatment with AP20187 significantly decreased the broken-up DFC phenotype (67%; P < 0.05) (G–J) A-tubulin (green) staining in ethanol-treated (G) or AP20187-treated (H) DFCcnpy1-MO+iFGFR1 embryos at the six-somite stage. (Scale bar: 20 µm.) (I and J) Number (I) or length (J) of KV primary cilia in ethanol-treated DFCcnpy1-MO+iFGFR1 (n = 9 or 36) or AP20187-treated DFCcnpy1-MO+iFGFR1 (n = 8 or 34) embryos at the six-somite stage. (Error bars show SEM.) Statistically significant (P < 0.05) differences could be seen in ethanol-treated versus AP20187-treated DFCcnpy1-MO+iFGFR1 embryos. (K) Percentages of cardiac laterality defect in ethanol-treated (n = 89) or AP20187-treated (n = 102) DFCcnpy1-MO+iFGFR1 embryos. The conditional activation of Fgfr1 after treatment with AP20187 alleviated the cardiac laterality defect (48%; P < 0.05).
|

