- Title
-
Evidence for a fizzled-mediated wnt pathway required for zebrafish dorsal mesoderm formation
- Authors
- Nasevicius, A., Hyatt, T., Kim, H., Guttman, J., Walsh, E., Sumanas, S., Wang, Y.S., and Ekker, S.C.
- Source
- Full text @ Development
|
Zebrafish frizzled A (fzA) protein and expression during day one of development. (A) Deduced zebrafish fzA protein sequence. The amino-terminal signaling sequence is in green, conservative cysteines are blue, and the putative PDZ binding motif is in red. Consensus N-linked glycosylation sites are indicated by orange, and the putative transmembrane domains are printed in bold italics. (BL) fzA distribution as determined using whole mount in situ hybridization. In each pairwise view of the same embryo, the arrowhead denotes dorsal or, in later embryos, anterior end of developing embryo as marked by fzA expression. (B,E) fzA localized just after the onset of zygotic transcription (sphere stage) to cells near yolk and animal cap boundary; lateral views with the animal pole to top. (C,F) By early gastrulation (shield stage), fzA is detected in the dorsal mesendoderm. e indicates epiblast and h indicates hypoblast. C is a dorsal and F a lateral view. (D,G) By the end of gastrulation, fzA expression is concentrated in the anterior part of the developing embryo. D is a rostral-dorsal view, G is a lateral view. (H,J) In mid-somitogenesis embryos, fzA is localized to rostral regions. H is a rostral-dorsal view with anterior to the top, J is a lateral view with anterior to the right. (I,K) During late somitogenesis fzA is localized to anterior ectoderm. p indicates protuberance and ov indicates optic vesicles. I is a rostral-dorsal view with anterior to the top, K a lateral view with anterior to the right. (L) Northern blot analysis of fzA mRNA expression. rRNA stained with ethidium bromide is shown in the lower panel as a loading control. In the upper panel, hybridization results are shown. Smaller (maternal) band is abundant at 1-4 cell stage and rapidly diminishes later in development. Expression of larger (zygotic) band is detected after zygotic transcription has initiated and continues throughout gastrulation and segmentation stages. Scale bars (B-G) (H-K) 250 μm. EXPRESSION / LABELING:
|
|
Misexpression of fz hyperdorsalizes zebrafish embryos. (A) Representation of fzA protein, as designated in Fig. 1A. B,E,H and L show comparably staged control embryos. C,F,I,J,M and N show fzA mRNA-injected embryos, stained in situ. (B,C) Animal pole view of shield stage embryos stained for GATA-2 (Detrich et al., 1995). The stained region is reduced in fzA-injected embryo. (E,F) Dorsal view of shield stage embryos stained for chordin (Schulter-Merker et al., 1997; Miller- Bertoglio et al, 1997). chordin expression is expanded in the injected embryo. (H,I) Dorsal view of 60% epiboly stage embryos stained for goosecoid (Stachel et al., 1993) which is expanded in the injected embryo. (J) Animal pole view of the same stage injected embryo stained for goosecoid. Note two stained regions corresponding to endogenous and ectopic shields. (L,M) Dorsal view of 75% epiboly embryos stained for the axial mesoderm marker sonic hedgehog (Krauss et al., 1993; Ekker et al., 1995). Note that staining is reduced in the fzAinjected embryo. (N) Lateral view of an embryo displaying ectopic axial structures, as visualized by sonic hedgehog staining. (D) 28 hour control and (G) fzA-injected embryo. Note reduced trunk and tail structures. (K) Lateral view of an ectopic axis generated as a result of HFZ5 misexpression. The primary (1°) axis is distinguished from the induced (2°) axis by the inheritance of the tracer GFP mRNA, as visualized by fluorescence (O) of the same living embryo. Scale bars, 250 μm. |
|
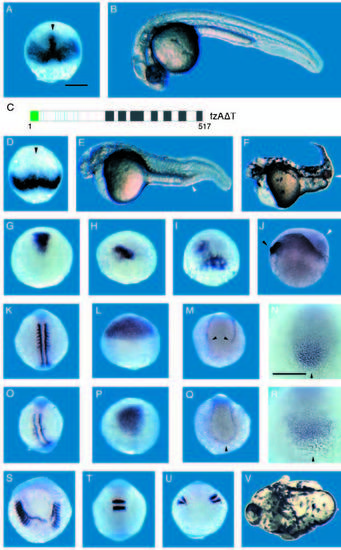
Misexpression of fzAΔT ventralizes zebrafish embryos. A,B,G,K,L,M,N and T are comparably staged control embryos. (C) Representation of fzAΔT, a dominant acting loss-of-function fz protein form that lacks the carboxy-terminal 60 amino acids (see Fig. 3). D-F, H-J, O-S and U,V show effects of fzAΔT misexpression. The dorsal mesoderm expression of chordin (arrowhead) is present in the control embryo (A) at approx. 65% epiboly but is missing in same stage fzAΔT-injected embryo (D). (E) 28-hour fzAΔT-injected embryo displaying a ventralized phenotype. Note extra cells in the tail (arrowhead) and reduced head structures. (F) Ventralized 2-day old fzAΔT-injected embryo. Note enlarged blood islands (arrowhead), a ventral-derived tissue. (G-J) Dorsal view of embryos stained for gsc. The stained region in fzAΔT-injected embryo (H) is reduced compared to control embryo (G). A significant fraction of fzAΔT-injected embryos showed mosaic gsc expression (I). (J) Lateral view of shield stage fzAΔT injected embryo, stained for gsc to mark the endogenous dorsoventral axis. Note thick cell layer (white arrowhead) observed during early gastrulation stages radially distant from the dorsal organizer (black arrowhead). (K,O) Dorsal view of mid-somitogenesis embryos stained for MyoD. Somitic mesoderm visualized by MyoD staining is reduced in fzAΔT-injected embryo (O) compared to control embryo (K); adaxial staining of MyoD is not significantly affected. (L,P) Dorsal view of 80% epiboly stage embryos stained for the anterior neuroectoderm marker otx-2. Note that otx-2 expression is significantly reduced in the fzAΔT-injected embryo (P) compared to control embryo (L). This phenotype is similar to the head reduction phenotype observed at 28 hours of development (E). (MN and Q-R). Ventral markers are expanded in fzAΔT-injected embryos, as judged by the tailbud expression of GATA-2 (M,Q) and eve-1 (N,R) in 10 somite embryos. In fzAΔT-injected embryos, ventral mesoderm stripes stained for GATA-2 are expanded and connected posteriorly (Q, black arrowhead) while in the uninjected embryo the stripes are separate (M, black arrowhead). eve-1 is expanded in fzAΔT-injected embryo (R) compared to control embryo (N). (S-V) High dose fzAΔT-injected embryos display a bifurcated axis phenotype distinct from effects on dorsoventral patterning (see text), which by 28 hours results in an embryo with only a single eye (V, white arrow indicates axis split). The phenotype can also be seen at the 10 somite stage when stained for MyoD (S) and Krox-20 (U). The stained areas are split into two distinct domains in fzAΔT-injected embryos compared to control embryos (K and T, accordingly). |
|
fz activity does not require putative PDZ binding motif. (A) Representation of fzAΔPBM, a fzA protein lacking the putative PDZ binding motif (see also Fig. 1A). Panels B-D and F-G show effects of fzΔPBM misexpression. (B) 28 hour embryo, showing dorsalized phenotype. Note reduced trunk and tail structures. (C, D) fzΔPBM induced axis duplication. Primary (1°) and secondary (2°) axes are indicated. y designates yolk. GFP is expressed mainly in the ectopic head (D). Expansion of (F), and ectopic (G) dorsal organizer tissue in fzΔPBM-injected embryos, as revealed by gsc expression in these 65% epiboly embryos |
|
Injection of wnt8 and Xgsk-3K85R hyperdorsalizes and Xgsk-3 ventralizes zebrafish embryos. (A-C) Dorsal view of control embryos stained for chordin (A; 65% epiboly), fzA (B; 65% epiboly) and gsc (C; 65% epiboly). wnt8-injected embryos display expanded expression of the dorsal mesoderm markers chordin (D, 60% epiboly), fzA (E, 65% epiboly) and gsc (F, 80% epiboly). Dorsal (G-I, K-L), animal (M) and lateral (N) views of embryos stained for chordin (G, K, shield stage), fzA (H, 65% epiboly, L, 70% epiboly), and gsc (I, 65% epiboly, M-N, 70% epiboly). (J,O,P) 28 hour embryos. (G-J) Embryos injected with Xgsk-3 mRNA. (G) Note lack of dorsal mesoderm expression of chordin (arrowhead; compare to Fig. 2E). (H,I) fzA and gsc expressions are reduced in Xsgk-3- injected embryos. (J) Note extra cells in the tail (arrowhead). (K-P) Embryos injected with Xgsk- 3K85R mRNA. (K-M) Note expanded chordin (K), fzA (L) and gsc (M) expression, phenotypes similar to those observed in wnt8 overexpression. N demonstrates that, unlike wnt8, Xgsk-3K85R can induce a new site of gsc expression de novo in zebrafish. (O) Dorsalized phenotype associated with reduction in body length and loss of tail structures. (P) Xgsk-3K85R injection results in a loss of head and eye structures, results similar to wnt8 misexpression in zebrafish (Kelly et al., 1995a,b). |