- Title
-
A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish
- Authors
- Riley, B.B., Zhu, C., Janetopoulos, C., and Aufderheide, K.J.
- Source
- Full text @ Dev. Biol.
|
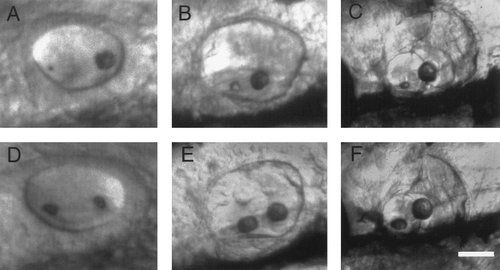
Early development of otoliths in wild-type and mnl embryos. Live embryos were viewed with a 100x objective using DIC optics. (A–C) Wild-type ear at 18.5 h (19 somites). (A) Lateral view of otic vesicle showing positions of anterior otolith and naked posterior tether. The anterior end of the vesicle has expanded more than the posterior end. (B) Close-up of the anterior end of the ear shown in A. The otolith, which appears as a composite of smaller particles, is attached to two distinct tethers. (C) Close-up of the posterior end of the ear shown in A. A single tether is visible, but no otolith is yet detectable. 20 min later (20 somites), the first signs of posterior otolith formation are evident (D). (E) Wild-type ear at 21.5 h (25 somites). Otoliths have increased in size and tethers have lengthened by several micrometers. Inset shows close-up of the anterior otolith and two tethers. (F) Wild-type ear at 24 h (30 somites). Otoliths have continued to grow. Inset shows close-up of anterior otolith and two tethers. No additional hair cell cilia are yet visible. (G) Close-up of mutant ear at 19 h (20 somites). Two tethers are visible but no signs of otolith formation are evident. (H) Mutant ear at 21.5 h (25 somites). Otoliths have not yet formed. Inset shows close-up of two anterior tethers lacking an otolith. (I) Mutant ear at 24 h (30 somites). A large posterior otolith is evident, but no anterior otolith has formed. Inset shows close-up of two anterior tethers lacking an otolith. All images are oriented with anterior to the left and dorsal upward. o, otoliths; arrows, tethers without otoliths. Scale bar, 5 (B, C, D, and G) or 25 μm (A, E, F, H, and I). Insets in E, F, H, and I show 2.6x enlargements of indicated features. |
|
Distinct classes of cilia inside the otic vesicle. Embryos were fixed and incubated with primary antibodies directed against acetylated tubulin followed by fluorescent secondary antibodies. Stained embryos were then imaged by confocal microscopy to determine the distribution of cilia in the otic vesicle. (A) 19-h ear showing >100 cilia. (B) 24-h ear with two sets of presumed tethers (arrows) and numerous shorter cilia. (C) 27-h ear with two sets of tethers (arrows). Most shorter cilia have disappeared. (D) 30-h ear showing two sets of tethers. Anterior tethers are attached to hair cells the cell bodies of which stain intensely for acetylated tubulin. Posterior hair cells also stain positively but occur at a deeper (more medial) focal plane than is shown. Scale bar, 25 μm. |
|
Sections of wild-type and mutant ears. Embryos were fixed and stained with peroxidase-conjugated antibodies to visualize acetylated tubulin and then sectioned. Specimens were lightly counterstained with eosin to help resolve cellular structure. (A) Phase-contrast image of the ear of a 19-h (20 somites) embryo showing numerous cilia. Although tethers cannot be clearly resolved at this time, all cells in the vicinity of the tether cells are columnar cells that fully span the otic epithelium, as indicated by the basal locations of their nuclei. (B) DIC image of the ear of a 20-h (22 somites) embryo showing two anterior tethers with apical otolith material (O). Tether cell bodies fully span the epithelium and show little or no staining for acetylated tubulin at this time. (C) Phase-contrast image of the ear of a 21.5-h (25 somites) embryo showing anterior otolith (O), tethers, and the overlapping bodies of two tether cells. The cytoplasm of tether cells counterstains less intensely than surrounding cells, a feature commonly observed in developing otic vesicles in both light and electron micrographs (Anniko, 1983; Noden and Van de Water, 1986; Hertwig and Schneider, 1986). The apical ends of tether cells stain positively for acetylated tubulin. (D) DIC image of the ear of a 21.5-h (25 somites) embryo showing anterior otolith (O), tethers, and tether cells. The distribution of acetylated tubulin at the apical ends of tether cells is clearly revealed in this lightly counterstained specimen. Anterior is to the right and dorsal is up in all panels. Scale bar, 20 (A), 15 (C), or 10 μm (B and D). |
|
Spontaneous and experimental fusion of otolith precursor particles. (A) DIC image of a mnl mutant ear at 22 h (26 somites). An untethered otolith-like mass rests on the medial surface (the lowest point) of the vesicle. A tethered posterior otolith is present but is not in focus in the image shown. (B–F) Phase-contrast images of the ear of a wild-type embryo at 21.5 h (25 somites). (B) The medial surface of the vesicle showing numerous small particles. Most particles are in rapid motion but those entering the beam of the laser tweezers are entrapped (arrowhead). Keeping the laser focused in the same spot for approximately 30 s captured additional particles, which fused into a single otolith-like mass. (C) The particle mass (arrowhead) remained whole after removal of the laser. (D and E) Close-ups showing the endogenous anterior and posterior otoliths, respectively. (F) Enhanced posterior otolith generated by using laser tweezers to collect particle masses from the medial surface and bringing them into contact with the endogenous otolith. Images in B–F were captured from a videotape of the tweezing process. Similar results were obtained in experiments performed on 20 wild-type and 15 mnl mutant embryos (not shown). In all panels, anterior is to the left and dorsal is upward. Scale bar, 24 (A), 17 (B and C), and 10 μm (D–F). |
|
Otolith development following modification. (A–C) Development of the same wild-type ear depicted in Figs. 5B–5F following enhancement of the posterior otolith with laser tweezers. (A) At 24 h, approximately 2 h after laser treatment, the posterior otolith is still many times the size of the anterior otolith. At 2 (B) and 6 days (C), the posterior otolith is nearly normal in size, but the anterior otolith remains much smaller than normal. (D–F) Development of a wild-type control embryo at 24 h (D), 2 days (E), and 6 days (F). Anterior is to the left and dorsal is upward in all panels. Scale bar, 25 (A and D), 45 (B and E), and 100 μm (C and F). |
|
Non-Brownian motion of otolith precursor particles. (A and B) Phase-contrast images captured on time-lapse video show two particles moving in a vortex (large circular arrow) just anterior and medial to the posterior otolith. Although the otolith is out of focus, its position can be discerned to the right of the vortex (asterisk). (A) Small arrows mark the initial positions of two particles orbiting on opposite sides of the vortex. (B) Approximately 0.1 s later, the particles have moved several micrometers in a clockwise orbit. The particles completed 10 – 12 rotations before being ejected from the vortex. Observation of this and other vortices revealed that small particles typically rotate faster than large particles, and that the overall speed of rotation fluctuates over time (not shown). Images were captured from a videotape of the same wild-type ear shown in Figs. 5B – 5F. Anterior is to the left and dorsal is upward. Scale bar, 5 μm. |
Reprinted from Developmental Biology, 191(2), Riley, B.B., Zhu, C., Janetopoulos, C., and Aufderheide, K.J., A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish, 191-201, Copyright (1997) with permission from Elsevier. Full text @ Dev. Biol.