- Title
-
Gliovascular transcriptional perturbations in Alzheimer's disease reveal molecular mechanisms of blood brain barrier dysfunction
- Authors
- İş, Ö., Wang, X., Reddy, J.S., Min, Y., Yilmaz, E., Bhattarai, P., Patel, T., Bergman, J., Quicksall, Z., Heckman, M.G., Tutor-New, F.Q., Can Demirdogen, B., White, L., Koga, S., Krause, V., Inoue, Y., Kanekiyo, T., Cosacak, M.I., Nelson, N., Lee, A.J., Vardarajan, B., Mayeux, R., Kouri, N., Deniz, K., Carnwath, T., Oatman, S.R., Lewis-Tuffin, L.J., Nguyen, T., Alzheimer’s Disease Neuroimaging Initiative, Carrasquillo, M.M., Graff-Radford, J., Petersen, R.C., Jr Jack, C.R., Kantarci, K., Murray, M.E., Nho, K., Saykin, A.J., Dickson, D.W., Kizil, C., Allen, M., Ertekin-Taner, N.
- Source
- Full text @ Nat. Commun.
|
Summary of the snRNAseq approach utilized in this study. |
|
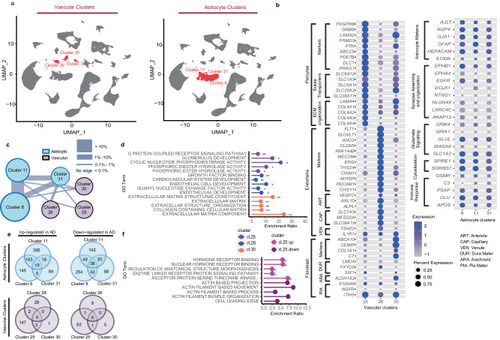
Vascular and astrocytic snRNAseq analyses reveal unique vascular clusters of which pericytes are the most perturbed in AD brains. |
|
Discovery, prioritization, validation, and replication of perturbed GVU vascular target-astrocyte ligand pair SMAD3-VEGFA. |
|
VEGF regulates of SMAD3 expression levels in pericytes. |
|
SMAD3-VEGF interactions influence blood-brain-barrier integrity in a zebrafish amyloidosis model. |