- Title
-
TANGO1 inhibitors reduce collagen secretion and limit tissue scarring
- Authors
- Raote, I., Rosendahl, A.H., Häkkinen, H.M., Vibe, C., Küçükaylak, I., Sawant, M., Keufgens, L., Frommelt, P., Halwas, K., Broadbent, K., Cunquero, M., Castro, G., Villemeur, M., Nüchel, J., Bornikoel, A., Dam, B., Zirmire, R.K., Kiran, R., Carolis, C., Andilla, J., Loza-Alvarez, P., Ruprecht, V., Jamora, C., Campelo, F., Krüger, M., Hammerschmidt, M., Eckes, B., Neundorf, I., Krieg, T., Malhotra, V.
- Source
- Full text @ Nat. Commun.
|
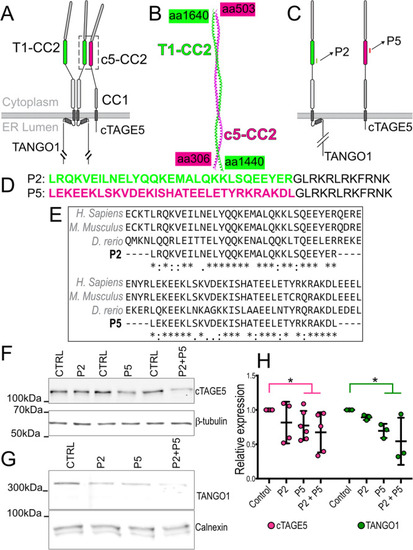
Design of peptides to inhibit TANGO1-cTAGE5 heterodimerization. |
|
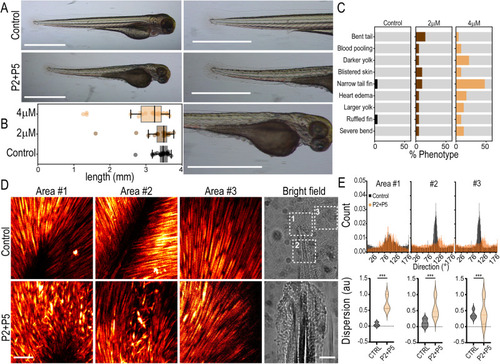
Peptides induce phenotypic changes in zebrafish consistent with TANGO1/cTAGE5 inhibition. |
|
Uptake of fluorescently labeled peptides into primary human dermal fibroblasts and inhibition of procollagen I secretion. |
|
Effects of peptide treatment on the secretome of human fibroblasts. |
|
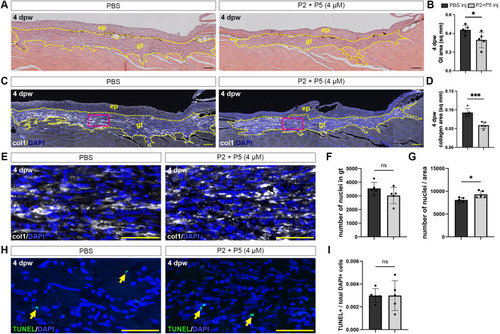
Peptides limit the formation of granulation tissue in zebrafish wounds. |
|
Peptides inhibit ECM protein secretion by TGFβ-activated healthy and scleroderma fibroblasts. |