- Title
-
Differentiation and functioning of the lateral line organ in zebrafish require Smpx activity
- Authors
- Diana, A., Ghilardi, A., Del Giacco, L.
- Source
- Full text @ Sci. Rep.
|
|
|
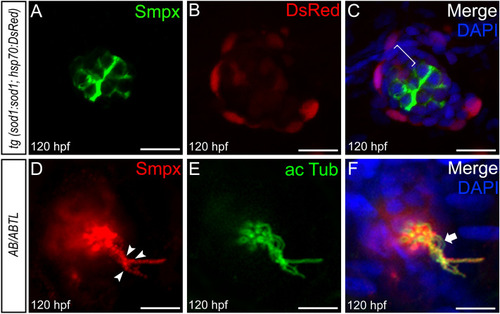
Smpx protein localization in the neuromast mechanosensory hair cells. Confocal Z-stacks of immunofluorescence on whole mount 120 hpf larvae. ( EXPRESSION / LABELING:
|
|
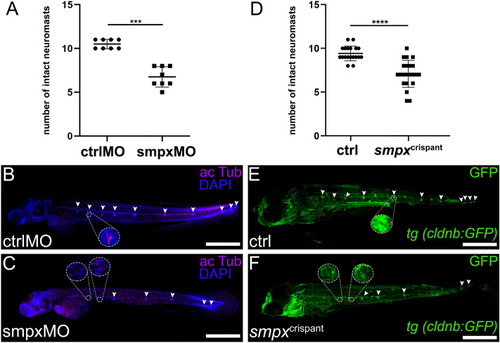
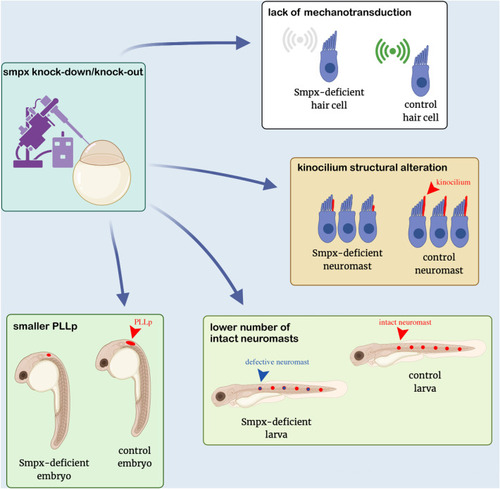
Smpx-deficiency leads to a lower number of intact neuromasts in morphant and crispant larvae. ( PHENOTYPE:
|
|
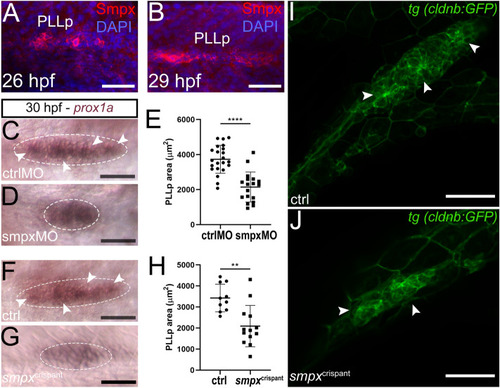
Smpx-deficiency causes the decrease in the PLLp size. ( |
|
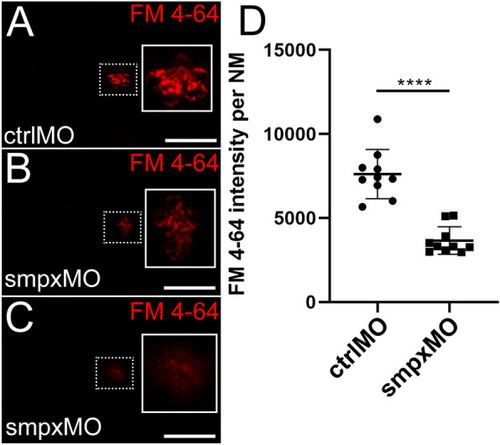
Smpx-deficiency causes a reduced mechanotransduction activity of the neuromast hair cells. ( PHENOTYPE:
|
|
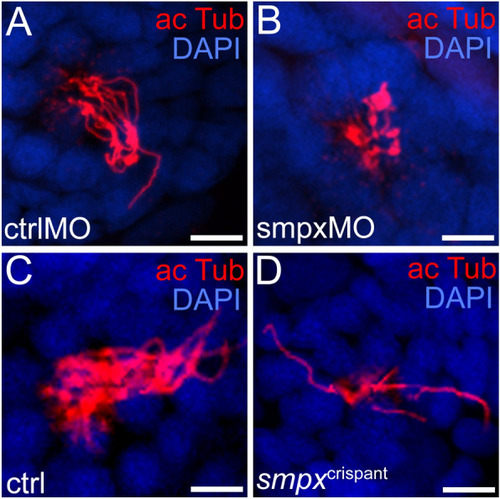
Smpx is required for normal kinocilium structure. Immunofluorescence performed on whole mount 120 hpf larvae with an antibody against acetylated tubulin to label the neuromast kinocilia. ( PHENOTYPE:
|
|
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

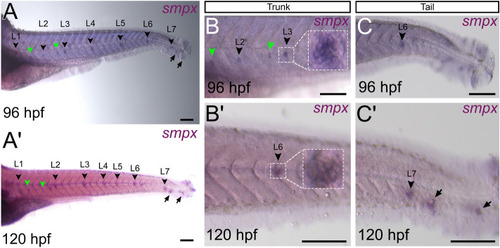
ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
|