- Title
-
Identification of chicken LOC420478 as Bucky ball equivalent and potential germ plasm organizer in birds
- Authors
- Klein, S., Dosch, R., Altgilbers, S., Kues, W.A.
- Source
- Full text @ Sci. Rep.
|
Regions of partial amino acid homology for chicken and zebrafish Bucky ball. ( |
|
High resolution confocal imaging of single channels for the reporter fluorescence (green fluorescence proteins, green label), for immunocytochemical Bucky ball reactivity in DF1 cells after transfection with different fusion plasmids (red label) and nuclear label using SIR (cyan label): ( |
|
Expression of Bucky ball after transfection of fusion plasmids in vitro and in embryonic stages around oviposition in vivo |
|
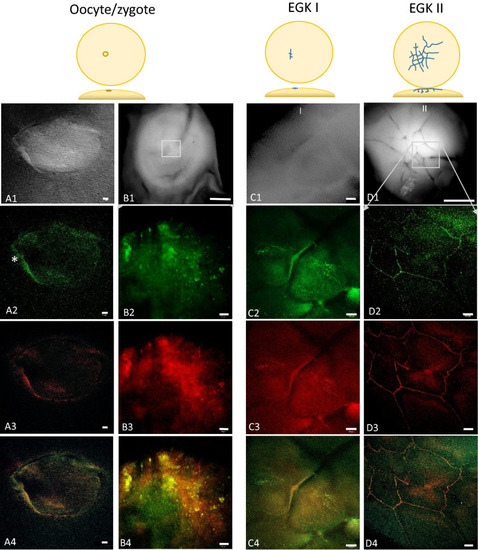
Accumulation of Bucky ball protein from the oocyte to early cleavage stages (EGK I/II) in chicken embryos. The upper panel represents differential interference ( |
|
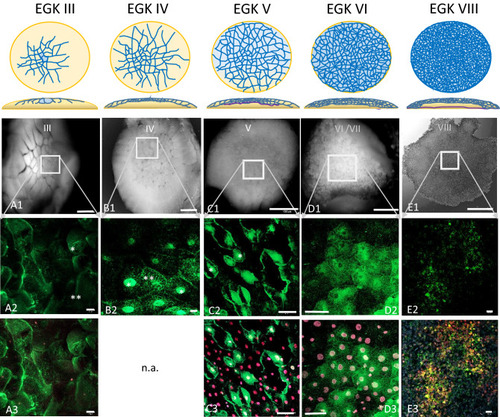
Dynamic localization of Bucky ball around major zygotic genome activation. The upper panel represents schemes for the formation of cells from stage EGK III, where first centrally located cells get separated to stage VIII, the second stage of area pellucida formation phase as described by Eyal-Giladi and Kochav |
|
Co-localization of Bucky ball and CVH during stages EK I to EK III. During the first two cleavage stages (EK I and EK III), Bucky ball strictly co-localized to CVH within the cleavage furrows and in condensed granules in the cleavage center A: ( |
|
Overview of an intrauterine embryo (EGK III) with two frames of the central region with Bucky ball immunolabeling in contrast to severely reduced immuno-fluorescence at the lateral outline of furrows. The two frames in the central region show the cells with Bucky ball immunofluorescence in cytoplasmic granular distribution. The lateral frame, documented at the exactly comparable acquisition conditions, does not provide signals above background. Scale = 500 µm. |
|
Co-localized Bucky ball and CVH expression in stage EGK IV. This composition of eight stitched images visualizes in the magnified center of a stage EGK IV embryo (frame of the overview in ( |
|
Bucky ball and CVH are no longer co-localized within the nuclear region of primordial germ cells during the |