- Title
-
Identification of ACTA2 as a Key Contributor to Venous Malformation
- Authors
- Wang, S., Zhou, Z., Li, J., Wang, Y., Li, H., Lv, R., Xu, G., Zhang, J., Bi, J., Huo, R.
- Source
- Full text @ Front Cell Dev Biol
|
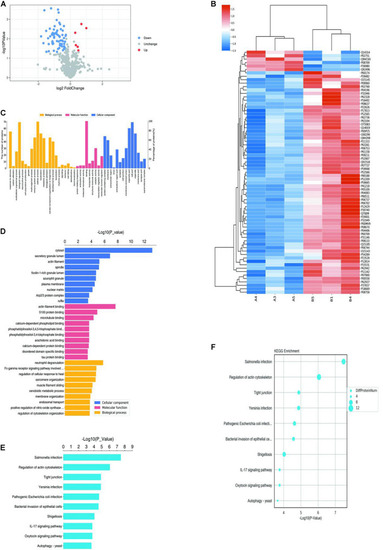
Proteomics and bioinformatics analysis of proteins involved in VM. |
|
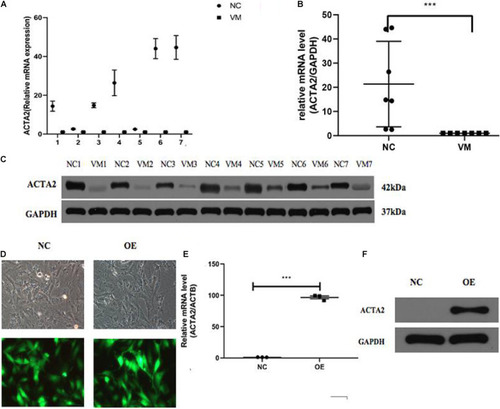
HCS screening identified ACTA2 as a critical gene in promoting VM proliferation. |
|
Lower expression of ACTA2 in VM tissues. |
|
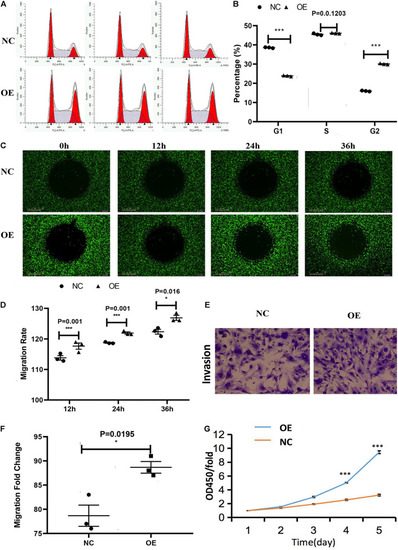
Effect of the overexpression of ACTA2 on the migration and invasion ability of HMEC-1. (A,B) Cell cycle distribution was determined by flow cytometry after propidium iodide (PI) staining. (C,D) OrisTM plate Wound-healing assay for the migration of HMEC1 after ACTA2 overexpressing. (E,F) Invasion assay for the invasion of HMEC1 after ACTA2 overexpressing. (G) CCK-8 assay for the proliferation of HMEC1 after ACTA2 overexpressing. *P < 0.05, **P < 0.01, ***P < 0.0001. |
|
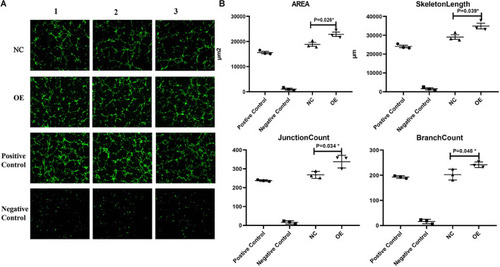
Effect of overexpression of ACTA2 on angiogenesis. |
|
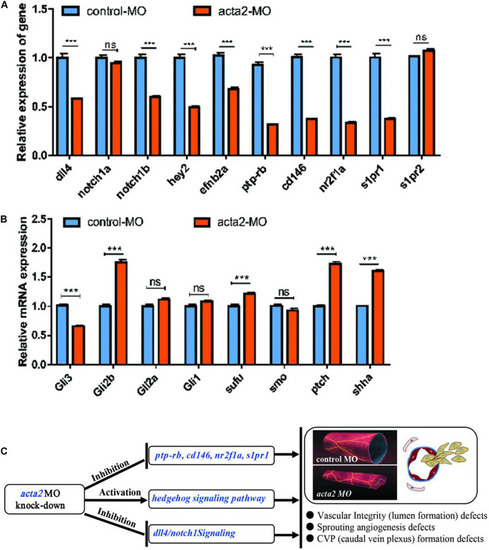
Effectiveness of acta2 knockdown was confirmed by RT-PCR and qRT-PCR. |
|
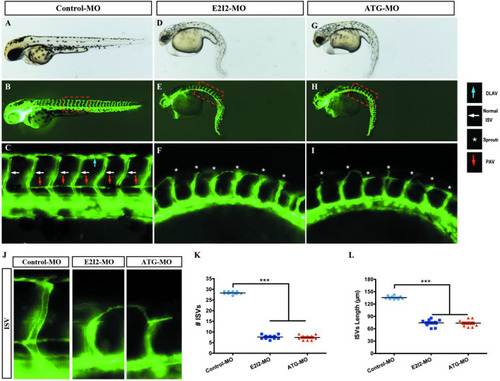
Morpholino knockdown of acta2 causes vascular defects. PHENOTYPE:
|
|
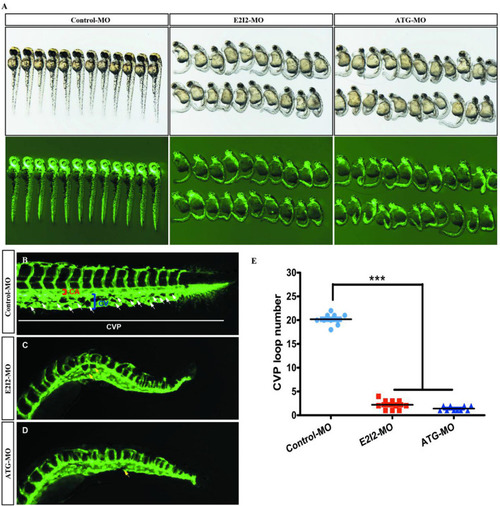
Acta2 knockdown impairs formation of the CVP in zebrafish. PHENOTYPE:
|
|
|